Interplay among cyclic diguanylate, HapR, and the general stress response regulator (RpoS) in the regulation of Vibrio cholerae hemagglutinin/protease
- PMID: 21965573
- PMCID: PMC3232884
- DOI: 10.1128/JB.05166-11
Interplay among cyclic diguanylate, HapR, and the general stress response regulator (RpoS) in the regulation of Vibrio cholerae hemagglutinin/protease
Abstract
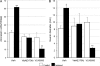
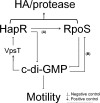
Vibrio cholerae secretes the Zn-dependent metalloprotease hemagglutinin (HA)/protease (mucinase), which is encoded by hapA and displays a broad range of potential pathogenic activities. Expression of HA/protease has a stringent requirement for the quorum-sensing regulator HapR and the general stress response regulator RpoS. Here we report that the second messenger cyclic diguanylic acid (c-di-GMP) regulates the production of HA/protease in a negative manner. Overexpression of a diguanylate cyclase to increase the cellular c-di-GMP pool resulted in diminished expression of HA/protease and its positive regulator, HapR. The effect of c-di-GMP on HapR was independent of LuxO but was abolished by deletion of the c-di-GMP binding protein VpsT, the LuxR-type regulator VqmA, or a single-base mutation in the hapR promoter that prevents autorepression. Though expression of HapR had a positive effect on RpoS biosynthesis, direct manipulation of the c-di-GMP pool at a high cell density did not significantly impact RpoS expression in the wild-type genetic background. In contrast, increasing the c-di-GMP pool severely inhibited RpoS expression in a ΔhapR mutant that is locked in a regulatory state mimicking low cell density. Based on the above findings, we propose a model for the interplay between HapR, RpoS, and c-di-GMP in the regulation of HA/protease expression.
Figures

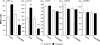



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

