Blood vessels restrain pancreas branching, differentiation and growth
- PMID: 21965615
- PMCID: PMC4496872
- DOI: 10.1242/dev.066548
Blood vessels restrain pancreas branching, differentiation and growth
Abstract
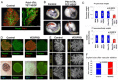
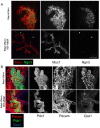
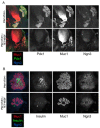
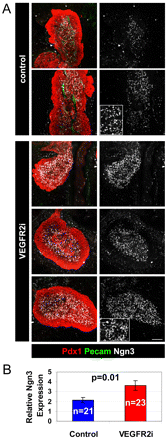
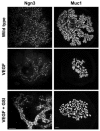
How organ size and form are controlled during development is a major question in biology. Blood vessels have been shown to be essential for early development of the liver and pancreas, and are fundamental to normal and pathological tissue growth. Here, we report that, surprisingly, non-nutritional signals from blood vessels act to restrain pancreas growth. Elimination of endothelial cells increases the size of embryonic pancreatic buds. Conversely, VEGF-induced hypervascularization decreases pancreas size. The growth phenotype results from vascular restriction of pancreatic tip cell formation, lateral branching and differentiation of the pancreatic epithelium into endocrine and acinar cells. The effects are seen both in vivo and ex vivo, indicating a perfusion-independent mechanism. Thus, the vasculature controls pancreas morphogenesis and growth by reducing branching and differentiation of primitive epithelial cells.
Figures


References
-
- Ahnfelt-Rønne J., Jørgensen M. C., Hald J., Madsen O. D., Serup P., Hecksher-Sørensen J. (2007b). An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J. Histochem. Cytochem. 55, 925-930. - PubMed
-
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877-881. - PubMed
-
- Butler J. M., Nolan D. J., Vertes E. L., Varnum-Finney B., Kobayashi H., Hooper A. T., Seandel M., Shido K., White I. A., Kobayashi M., et al. (2010b). Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251-264. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

