Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36Ra) activity
- PMID: 21965679
- PMCID: PMC3234937
- DOI: 10.1074/jbc.M111.267922
Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36Ra) activity
Abstract
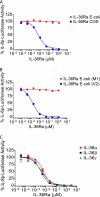
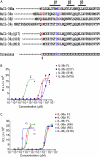
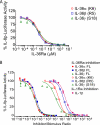
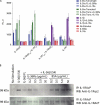
IL-36α, IL-36β, and IL-36γ (formerly IL-1F6, IL-1F8, and IL-1F9) are IL-1 family members that signal through the IL-1 receptor family members IL-1Rrp2 (IL-1RL2) and IL-1RAcP. IL-36Ra (formerly IL-1F5) has been reported to antagonize IL-36γ. However, our previous attempts to demonstrate IL-36Ra antagonism were unsuccessful. Here, we demonstrate that IL-36Ra antagonist activity is dependent upon removal of its N-terminal methionine. IL-36Ra starting at Val-2 is fully active and capable of inhibiting not only IL-36γ but also IL-36α and IL-36β. Val-2 of IL-36Ra lies 9 amino acids N-terminal to an A-X-Asp motif conserved in all IL-1 family members. In further experiments, we show that truncation of IL-36α, IL-36β, and IL-36γ to this same point increased their specific activity by ∼10(3)-10(4)-fold (from EC(50) 1 μg/ml to EC(50) 1 ng/ml). Inhibition of truncated IL-36β activity required ∼10(2)-10(3)-fold excess IL-36Ra, similar to the ratio required for IL-1Ra to inhibit IL-1β. Chimeric receptor experiments demonstrated that the extracellular (but not cytoplasmic) domain of IL-1Rrp2 or IL-1R1 is required for inhibition by their respective natural antagonists. IL-36Ra bound to IL-1Rrp2, and pretreatment of IL-1Rrp2-expressing cells with IL-36Ra prevented IL-36β-mediated co-immunoprecipitation of IL-1Rrp2 with IL-1RAcP. Taken together, these results suggest that the mechanism of IL-36Ra antagonism is analogous to that of IL-1Ra, such that IL-36Ra binds to IL-1Rrp2 and prevents IL-1RAcP recruitment and the formation of a functional signaling complex. In addition, truncation of IL-36α, IL-36β, and IL-36γ dramatically enhances their activity, suggesting that post-translational processing is required for full activity.
Figures
References
-
- Dunn E., Sims J. E., Nicklin M. J., O'Neill L. A. (2001) Trends Immunol. 22, 533–536 - PubMed
-
- Sims J. E., Smith D. E. (2010) Nat. Rev. Immunol. 10, 89–102 - PubMed
-
- O'Neill L. A. (2008) Immunol. Rev. 226, 10–18 - PubMed
-
- Greenfeder S. A., Nunes P., Kwee L., Labow M., Chizzonite R. A., Ju G. (1995) J. Biol. Chem. 270, 13757–13765 - PubMed
-
- Born T. L., Thomassen E., Bird T. A., Sims J. E. (1998) J. Biol. Chem. 273, 29445–29450 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

