TGF-beta regulation of focal adhesion proteins and motility of premalignant oral lesions via protein phosphatase 1
- PMID: 21965722
- PMCID: PMC3622218
TGF-beta regulation of focal adhesion proteins and motility of premalignant oral lesions via protein phosphatase 1
Abstract
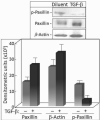
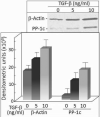
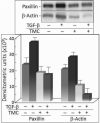
Premalignant oral lesions have a high incidence of recurrence and progression to malignant disease and, although studies have shown the contribution of transforming growth factor β (TGF-β) to cancer progression, none have been conducted with premalignant oral lesion cells to determine the impact of TGF-β in stimulating properties that are characteristic of more invasive cells. The present study focused on TGF-β-modulation of paxillin and the serine/threonine protein phosphatase PP-1, and the impact on cellular motility. These studies show that TGF-β stimulates premalignant lesion cell motility and up regulates expression of paxillin, as well as its co-localization with PP-1, while concurrently diminishing the level of paxillin serine phosphorylation. The TGF-β-mediated up regulation of paxillin and co-localization with actin, as well as the TGF-β-stimulated motility of premalignant lesion cells, were all blocked by inhibiting PP-1, indicating their dependence on PP-1 activity. These studies suggest interplay between TGF-β and PP-1 in promoting a more malignant phenotype in premalignant oral lesion cells.
Figures


Similar articles
-
Interrelationship between protein phosphatase 1 and TGF-{beta} in regulating motility and cytoskeletal architecture of endothelial cells.Anticancer Res. 2010 Dec;30(12):4861-6. Anticancer Res. 2010. PMID: 21187463 Free PMC article.
-
Activin A and TGF-beta stimulate phosphorylation of focal adhesion proteins and cytoskeletal reorganization in rat aortic smooth muscle cells.Exp Cell Res. 1999 Aug 25;251(1):194-202. doi: 10.1006/excr.1999.4573. Exp Cell Res. 1999. PMID: 10438585
-
Protein phosphatase-2A restricts migration of Lewis lung carcinoma cells by modulating the phosphorylation of focal adhesion proteins.Int J Cancer. 2003 Jan 1;103(1):38-44. doi: 10.1002/ijc.10772. Int J Cancer. 2003. PMID: 12455051
-
c-Fos accelerates hepatocyte conversion to a fibroblastoid phenotype through ERK-mediated upregulation of paxillin-Serine178 phosphorylation.Mol Carcinog. 2009 Jun;48(6):532-44. doi: 10.1002/mc.20492. Mol Carcinog. 2009. PMID: 18973190
-
Paxillin: a crossroad in pathological cell migration.J Hematol Oncol. 2017 Feb 18;10(1):50. doi: 10.1186/s13045-017-0418-y. J Hematol Oncol. 2017. PMID: 28214467 Free PMC article. Review.
Cited by
-
Human ortholog of Drosophila Melted impedes SMAD2 release from TGF-β receptor I to inhibit TGF-β signaling.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):E3000-9. doi: 10.1073/pnas.1504671112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26039994 Free PMC article.
-
Repurposing mesalazine against cardiac fibrosis in vitro.Naunyn Schmiedebergs Arch Pharmacol. 2021 Mar;394(3):533-543. doi: 10.1007/s00210-020-01998-9. Epub 2020 Oct 16. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33064167 Free PMC article.
-
TGF-β1 signalling in Alzheimer's pathology and cytoskeletal reorganization: a specialized Tau perspective.J Neuroinflammation. 2023 Mar 13;20(1):72. doi: 10.1186/s12974-023-02751-8. J Neuroinflammation. 2023. PMID: 36915196 Free PMC article. Review.
-
Inflammatory Mediators in Oral Cancer: Pathogenic Mechanisms and Diagnostic Potential.Front Oral Health. 2021 Feb 22;2:642238. doi: 10.3389/froh.2021.642238. eCollection 2021. Front Oral Health. 2021. PMID: 35047997 Free PMC article. Review.
-
Exploring prognostic value and regulation network of PPP1R1A in hepatocellular carcinoma.Hum Cell. 2022 Nov;35(6):1856-1868. doi: 10.1007/s13577-022-00771-9. Epub 2022 Aug 26. Hum Cell. 2022. PMID: 36018458
References
-
- Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-β1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66:9583–9590. - PubMed
-
- Li Y, Yang K, Mao Q, Zheng X, Kong D, Xie L. Inhibition of TGF-β receptor I by siRNA suppresses the motility and invasiveness of T24 bladder cancer cells via modulation of integrins and matrix metalloproteinase. Int Urol Nephrol. 2010;42:315–323. - PubMed
-
- Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J Cell Sci. 2011;124:2220–2230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
