Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects
- PMID: 21966064
- PMCID: PMC3173578
- DOI: 10.5665/SLEEP.1272
Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects
Abstract
Study objectives: To evaluate the next-morning residual effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects following bedtime dosing and a middle of the night awakening.
Design: Single-center, randomized, double-blind, double-dummy, placebo-controlled, crossover study.
Setting: Utrecht University, The Netherlands.
Participants: 30 healthy volunteers (15 males and 15 females).
Interventions: a single dose of ramelteon (8 mg), zopiclone (7.5 mg), and placebo, administered at bedtime.
Measurements: A balance test was performed at night. Other tests were performed the following morning, 8.5 h after administration. Subjects performed a 100-km highway driving test in normal traffic. Primary outcome measure was the standard deviation of the lateral position (SDLP), i.e., the weaving of the car. After driving, cognitive, memory, and psychomotor tests were performed and mood was assessed.
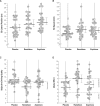
Results: SDLP was significantly increased after the intake of ramelteon (+2.2 cm) and zopiclone (+2.9 cm). Ramelteon and zopiclone produced significant impairment on reaction time (P<0.024) in the Sternberg Memory Scanning Test, slow (P<0.007) and fast (P<0.010) tracking, reaction speed (P<0.015) and tracking (P<0.001) in the Divided Attention Test, and delayed recall (P<0.032) in the Word Learning Test. In contrast to ramelteon, zopiclone additionally impaired performance on the Digit Symbol Substitution Test (P<0.001) and the balance test (P<0.001).
Conclusions: Ramelteon (8 mg) and zopiclone (7.5 mg) significantly impaired driving performance, cognitive, memory, and psychomotor performance the morning following bedtime administration. In contrast to zopiclone, ramelteon produced no balance impairments. CLINICAL TRIAL IDENTIFIER: NCT00319215 (www.clinicaltrials.gov).
Keywords: Driving; balance; memory; psychomotor; ramelteon; zopiclone.
Figures

Comment in
-
The challenges of interpreting residual effects of hypnotics.Sleep. 2011 Oct 1;34(10):1285-6. doi: 10.5665/SLEEP.1258. Sleep. 2011. PMID: 21966057 Free PMC article. No abstract available.
References
-
- Ebert B, Wafford KA, Deacon S. Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–29. - PubMed
-
- Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev. 2004;8:309–25. - PubMed
-
- Verster JC, Veldhuijzen DS, Patat A, Olivier B, Volkerts ER. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63–71. - PubMed
-
- Neutel CI. Risk of traffic accident injury after a prescription for a benzodiazepine. Ann Epidemiol. 1995;5:239–244. - PubMed
-
- Hemmelgarn B, Suissa S, Huang A, Biovin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278:27–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

