Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing
- PMID: 21966073
- PMCID: PMC3174843
- DOI: 10.5665/SLEEP.1290
Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing
Abstract
Study objectives: Thalamo-cortical spindles driven by the up-state of neocortical slow (< 1 Hz) oscillations (SOs) represent a candidate mechanism of memory consolidation during sleep. We examined interactions between SOs and spindles in human slow wave sleep, focusing on the presumed existence of 2 kinds of spindles, i.e., slow frontocortical and fast centro-parietal spindles.
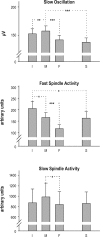
Design: Two experiments were performed in healthy humans (24.5 ± 0.9 y) investigating undisturbed sleep (Experiment I) and the effects of prior learning (word paired associates) vs. non-learning (Experiment II) on multichannel EEG recordings during sleep.
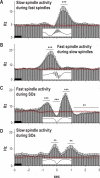
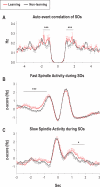
Measurements and results: Only fast spindles (12-15 Hz) were synchronized to the depolarizing SO up-state. Slow spindles (9-12 Hz) occurred preferentially at the transition into the SO down-state, i.e., during waning depolarization. Slow spindles also revealed a higher probability to follow rather than precede fast spindles. For sequences of individual SOs, fast spindle activity was largest for "initial" SOs, whereas SO amplitude and slow spindle activity were largest for succeeding SOs. Prior learning enhanced this pattern.
Conclusions: The finding that fast and slow spindles occur at different times of the SO cycle points to disparate generating mechanisms for the 2 kinds of spindles. The reported temporal relationships during SO sequences suggest that fast spindles, driven by the SO up-state feed back to enhance the likelihood of succeeding SOs together with slow spindles. By enforcing such SO-spindle cycles, particularly after prior learning, fast spindles possibly play a key role in sleep-dependent memory processing.
Keywords: Human; memory; sleep spindles; slow oscillations.
Figures



References
-
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

