Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia
- PMID: 21966075
- PMCID: PMC3174845
- DOI: 10.5665/SLEEP.1294
Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia
Abstract
Study objectives: To evaluate the efficacy and safety of doxepin (DXP) 3 mg and 6 mg in adults diagnosed with primary insomnia.
Design and methods: The study was a randomized, double-blind, parallel-group, placebo-controlled trial. Patients meeting DSM-IV-TR criteria for primary insomnia were randomized to 35 days of nightly treatment with DXP 3 mg (n=75), DXP 6 mg (n=73), or placebo (PBO; n=73), followed by 2 nights of single-blind PBO to evaluate discontinuation (DC) effects. Efficacy was assessed using polysomnography (PSG) and patient reports. Efficacy data were examined for Night (N) 1, N15, and N29. Safety assessments were conducted throughout the study.
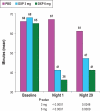
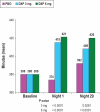
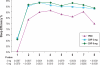
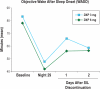
Results: Compared with PBO, DXP 3 and 6 mg significantly improved wake time after sleep onset (WASO) on N1 (3 mg and 6 mg; P<0.0001), N15 (3 mg P=0.0025; 6 mg P=0.0009), and N29 (3 mg P=0.0248; 6 mg P=0.0009), latency to persistent sleep (LPS) on N1 (3 mg P=0.0047; 6 mg P=0.0007), and total sleep time (TST) on N1 (3 mg and 6 mg P<0.0001), N15 (6 mg P=0.0035), and N29 (3 mg P=0.0261; 6 mg P<0.0001). In terms of early morning awakenings, DXP 3 and 6 mg demonstrated significant improvements in SE in the final quarter of the night on N1, N15, and N29, with the exception of 3 mg on N29 (P=0.0691). Rates of discontinuation were low, and the safety profiles were comparable across the 3 treatment groups. There were no significant next-day residual effects, and there were no spontaneous reports of memory impairment, complex sleep behaviors, anticholinergic effects, weight gain, or increased appetite. Additionally, there was no evidence of rebound insomnia after DXP discontinuation.
Conclusions: Five weeks of nightly administration of DXP 3 mg and 6 mg to adults with chronic primary insomnia resulted in significant and sustained improvements in sleep maintenance and early morning awakenings (with the exception of SE in the final quarter of the night on N29 for 3 mg [P=0.0691]). These sleep improvements were not accompanied by next-day residual effects or followed by rebound insomnia or withdrawal effects upon discontinuation. These findings confirm the unique profile of sleep maintenance efficacy and safety of DXP observed in prior studies.
Keywords: Chronic insomnia; TST; WASO; WTDS; doxepin; early morning awakenings; sleep maintenance insomnia.
Figures
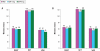
References
-
- Lichstein KL, Durrence HH, Taylor DJ, et al. Epidemiology of sleep: age, gender, and ethnicity. Mahwah, NJ: Erlbam; 2004.
-
- Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 2004;39:411–8. - PubMed
-
- Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31:333–46. - PubMed
-
- Buysse D, Reynolds CF, III, Kupfer DJ, et al. Effects of diagnosis on treatment recommendations in chronic insomnia-a report from the APA/NIMH DSM-IV field trial. Sleep. 1997;20:542–52. - PubMed
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Association; 1997. text revision (DSM-IV-TR)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

