Destruction of lymphoid organ architecture and hepatitis caused by CD4+ T cells
- PMID: 21966366
- PMCID: PMC3179489
- DOI: 10.1371/journal.pone.0024772
Destruction of lymphoid organ architecture and hepatitis caused by CD4+ T cells
Abstract
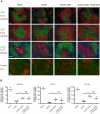
Immune responses have the important function of host defense and protection against pathogens. However, the immune response also causes inflammation and host tissue injury, termed immunopathology. For example, hepatitis B and C virus infection in humans cause immunopathological sequel with destruction of liver cells by the host's own immune response. Similarly, after infection with lymphocytic choriomeningitis virus (LCMV) in mice, the adaptive immune response causes liver cell damage, choriomeningitis and destruction of lymphoid organ architecture. The immunopathological sequel during LCMV infection has been attributed to cytotoxic CD8(+) T cells. However, we now show that during LCMV infection CD4(+) T cells selectively induced the destruction of splenic marginal zone and caused liver cell damage with elevated serum alanin-transferase (ALT) levels. The destruction of the splenic marginal zone by CD4(+) T cells included the reduction of marginal zone B cells, marginal zone macrophages and marginal zone metallophilic macrophages. Functionally, this resulted in an impaired production of neutralizing antibodies against LCMV. Furthermore, CD4(+) T cells reduced B cells with an IgM(high)IgD(low) phenotype (transitional stage 1 and 2, marginal zone B cells), whereas other B cell subtypes such as follicular type 1 and 2 and germinal center/memory B cells were not affected. Adoptive transfer of CD4(+) T cells lacking different important effector cytokines and cytolytic pathways such as IFNγ, TNFα, perforin and Fas-FasL interaction did reveal that these cytolytic pathways are redundant in the induction of immunopathological sequel in spleen. In conclusion, our results define an important role of CD4(+) T cells in the induction of immunopathology in liver and spleen. This includes the CD4(+) T cell mediated destruction of the splenic marginal zone with consecutively impaired protective neutralizing antibody responses.
Conflict of interest statement
Figures

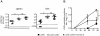




References
-
- Zinkernagel RM, Haenseler E, Leist T, Cerny A, Hengartner H, et al. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986;164:1075–1092. - PMC - PubMed
-
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. - PubMed
-
- Matter MS, Ochsenbein AF. Natural antibodies target virus-antibody complexes to organized lymphoid tissue. Autoimmun Rev. 2008;7:480–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

