Plexin-B2 negatively regulates macrophage motility, Rac, and Cdc42 activation
- PMID: 21966369
- PMCID: PMC3179467
- DOI: 10.1371/journal.pone.0024795
Plexin-B2 negatively regulates macrophage motility, Rac, and Cdc42 activation
Abstract
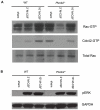
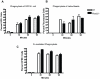
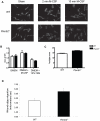
Plexins are cell surface receptors widely studied in the nervous system, where they mediate migration and morphogenesis though the Rho family of small GTPases. More recently, plexins have been implicated in immune processes including cell-cell interaction, immune activation, migration, and cytokine production. Plexin-B2 facilitates ligand induced cell guidance and migration in the nervous system, and induces cytoskeletal changes in overexpression assays through RhoGTPase. The function of Plexin-B2 in the immune system is unknown. This report shows that Plexin-B2 is highly expressed on cells of the innate immune system in the mouse, including macrophages, conventional dendritic cells, and plasmacytoid dendritic cells. However, Plexin-B2 does not appear to regulate the production of proinflammatory cytokines, phagocytosis of a variety of targets, or directional migration towards chemoattractants or extracellular matrix in mouse macrophages. Instead, Plxnb2(-/-) macrophages have greater cellular motility than wild type in the unstimulated state that is accompanied by more active, GTP-bound Rac and Cdc42. Additionally, Plxnb2(-/-) macrophages demonstrate faster in vitro wound closure activity. Studies have shown that a closely related family member, Plexin-B1, binds to active Rac and sequesters it from downstream signaling. The interaction of Plexin-B2 with Rac has only been previously confirmed in yeast and bacterial overexpression assays. The data presented here show that Plexin-B2 functions in mouse macrophages as a negative regulator of the GTPases Rac and Cdc42 and as a negative regulator of basal cell motility and wound healing.
Conflict of interest statement
Figures

Similar articles
-
Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho.Curr Biol. 2001 Mar 6;11(5):339-44. doi: 10.1016/s0960-9822(01)00092-6. Curr Biol. 2001. PMID: 11267870
-
Plexin-B2 and Plexin-D1 in dendritic cells: expression and IL-12/IL-23p40 production.PLoS One. 2012;7(8):e43333. doi: 10.1371/journal.pone.0043333. Epub 2012 Aug 15. PLoS One. 2012. PMID: 22916243 Free PMC article.
-
Plexin D1 negatively regulates macrophage-derived foam cell migration via the focal adhesion kinase/Paxillin pathway.Biochem Biophys Res Commun. 2024 Sep 17;725:150236. doi: 10.1016/j.bbrc.2024.150236. Epub 2024 Jun 6. Biochem Biophys Res Commun. 2024. PMID: 38897039
-
The role of Rho GTPases' substrates Rac and Cdc42 in osteoclastogenesis and relevant natural medicinal products study.Biosci Rep. 2020 Jul 31;40(7):BSR20200407. doi: 10.1042/BSR20200407. Biosci Rep. 2020. PMID: 32578854 Free PMC article. Review.
-
Plexin B2 in physiology and pathophysiology of the central nervous system.Int Immunopharmacol. 2025 May 16;155:114627. doi: 10.1016/j.intimp.2025.114627. Epub 2025 Apr 13. Int Immunopharmacol. 2025. PMID: 40220620 Review.
Cited by
-
Genome-wide association study of antibody response to Newcastle disease virus in chicken.BMC Genet. 2013 May 10;14:42. doi: 10.1186/1471-2156-14-42. BMC Genet. 2013. PMID: 23663563 Free PMC article.
-
The Role of Semaphorins and Their Receptors in Innate Immune Responses and Clinical Diseases of Acute Inflammation.Front Immunol. 2021 May 3;12:672441. doi: 10.3389/fimmu.2021.672441. eCollection 2021. Front Immunol. 2021. PMID: 34012455 Free PMC article. Review.
-
Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile.Bioact Mater. 2022 Aug 2;25:732-747. doi: 10.1016/j.bioactmat.2022.07.004. eCollection 2023 Jul. Bioact Mater. 2022. PMID: 37056276 Free PMC article.
-
Experimental validation of 5 in-silico predicted glioma biomarkers.Neuro Oncol. 2013 Dec;15(12):1625-34. doi: 10.1093/neuonc/not124. Epub 2013 Oct 24. Neuro Oncol. 2013. PMID: 24158112 Free PMC article.
-
A Comparative Biology of Microglia Across Species.Front Cell Dev Biol. 2021 Apr 1;9:652748. doi: 10.3389/fcell.2021.652748. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869210 Free PMC article. Review.
References
-
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. - PubMed
-
- Ohta K, Takagi S, Asou H, Fujisawa H. Involvement of neuronal cell surface molecule B2 in the formation of retinal plexiform layers. Neuron. 1992;9:151–161. - PubMed
-
- Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, et al. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14:1189–1199. - PubMed
-
- Serini G, Maione F, Giraudo E, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12:187–193. - PubMed
-
- O'Connor BP, Ting JP. The evolving role of semaphorins and plexins in the immune system: Plexin-A1 regulation of dendritic cell function. Immunol Res. 2008;41:217–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

