The A-current modulates learning via NMDA receptors containing the NR2B subunit
- PMID: 21966384
- PMCID: PMC3180285
- DOI: 10.1371/journal.pone.0024915
The A-current modulates learning via NMDA receptors containing the NR2B subunit
Abstract
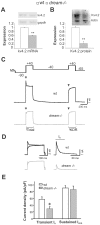
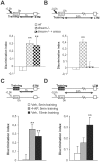
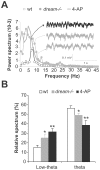
Synaptic plasticity involves short- and long-term events, although the molecular mechanisms that underlie these processes are not fully understood. The transient A-type K(+) current (I(A)) controls the excitability of the dendrites from CA1 pyramidal neurons by regulating the back-propagation of action potentials and shaping synaptic input. Here, we have studied how decreases in I(A) affect cognitive processes and synaptic plasticity. Using wild-type mice treated with 4-AP, an I(A) inhibitor, and mice lacking the DREAM protein, a transcriptional repressor and modulator of the I(A), we demonstrate that impairment of I(A) decreases the stimulation threshold for learning and the induction of early-LTP. Hippocampal electrical recordings in both models revealed alterations in basal electrical oscillatory properties toward low-theta frequencies. In addition, we demonstrated that the facilitated learning induced by decreased I(A) requires the activation of NMDA receptors containing the NR2B subunit. Together, these findings point to a balance between the I(A) and the activity of NR2B-containing NMDA receptors in the regulation of learning.
Conflict of interest statement
Figures


Similar articles
-
Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats.PLoS One. 2009 Oct 19;4(10):e7486. doi: 10.1371/journal.pone.0007486. PLoS One. 2009. PMID: 19838302 Free PMC article.
-
Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors.J Neurosci. 2016 Nov 9;36(45):11521-11531. doi: 10.1523/JNEUROSCI.1519-16.2016. J Neurosci. 2016. PMID: 27911756 Free PMC article.
-
Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling.PLoS One. 2010 Jul 22;5(7):e11732. doi: 10.1371/journal.pone.0011732. PLoS One. 2010. PMID: 20661302 Free PMC article.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Muscarinic Receptors, from Synaptic Plasticity to its Role in Network Activity.Neuroscience. 2021 Feb 21;456:60-70. doi: 10.1016/j.neuroscience.2020.04.005. Epub 2020 Apr 8. Neuroscience. 2021. PMID: 32278062 Review.
Cited by
-
Neuroplasticity of A-type potassium channel complexes induced by chronic alcohol exposure enhances dendritic calcium transients in hippocampus.Psychopharmacology (Berl). 2015 Jun;232(11):1995-2006. doi: 10.1007/s00213-014-3835-4. Epub 2014 Dec 17. Psychopharmacology (Berl). 2015. PMID: 25510858 Free PMC article.
-
Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring.Eur J Neurosci. 2014 May;39(10):1558-71. doi: 10.1111/ejn.12535. Epub 2014 Mar 19. Eur J Neurosci. 2014. PMID: 24646396 Free PMC article.
-
The HERC1 E3 Ubiquitin Ligase is essential for normal development and for neurotransmission at the mouse neuromuscular junction.Cell Mol Life Sci. 2015 Aug;72(15):2961-71. doi: 10.1007/s00018-015-1878-2. Epub 2015 Mar 8. Cell Mol Life Sci. 2015. PMID: 25746226 Free PMC article.
-
Identification of a Novel Rat NR2B Subunit Gene Promoter Region Variant and Its Association with Microwave-Induced Neuron Impairment.Mol Neurobiol. 2016 May;53(4):2100-11. doi: 10.1007/s12035-015-9169-3. Epub 2015 Apr 28. Mol Neurobiol. 2016. PMID: 25917873
-
Chronic Administration of Benzo(a)pyrene Induces Memory Impairment and Anxiety-Like Behavior and Increases of NR2B DNA Methylation.PLoS One. 2016 Feb 22;11(2):e0149574. doi: 10.1371/journal.pone.0149574. eCollection 2016. PLoS One. 2016. PMID: 26901155 Free PMC article.
References
-
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. - PubMed
-
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. - PubMed
-
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. - PubMed
-
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

