GW501516, a PPARδ agonist, ameliorates tubulointerstitial inflammation in proteinuric kidney disease via inhibition of TAK1-NFκB pathway in mice
- PMID: 21966476
- PMCID: PMC3178624
- DOI: 10.1371/journal.pone.0025271
GW501516, a PPARδ agonist, ameliorates tubulointerstitial inflammation in proteinuric kidney disease via inhibition of TAK1-NFκB pathway in mice
Abstract
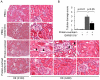
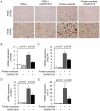
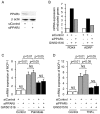
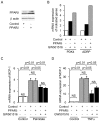
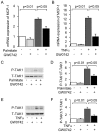
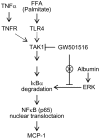
Peroxisome proliferator-activated receptors (PPARs) are a nuclear receptor family of ligand-inducible transcription factors, which have three different isoforms: PPARα, δ and γ. It has been demonstrated that PPARα and γ agonists have renoprotective effects in proteinuric kidney diseases; however, the role of PPARδ agonists in kidney diseases remains unclear. Thus, we examined the renoprotective effect of GW501516, a PPARδ agonist, in a protein-overload mouse nephropathy model and identified its molecular mechanism. Mice fed with a control diet or GW501516-containing diet were intraperitoneally injected with free fatty acid (FFA)-bound albumin or PBS(-). In the control group, protein overload caused tubular damages, macrophage infiltration and increased mRNA expression of MCP-1 and TNFα. These effects were prevented by GW501516 treatment. In proteinuric kidney diseases, excess exposure of proximal tubular cells to albumin, FFA bound to albumin or cytokines such as TNFα is detrimental. In vitro studies using cultured proximal tubular cells showed that GW501516 attenuated both TNFα- and FFA (palmitate)-induced, but not albumin-induced, MCP-1 expression via direct inhibition of the TGF-β activated kinase 1 (TAK1)-NFκB pathway, a common downstream signaling pathway to TNFα receptor and toll-like receptor-4. In conclusion, we demonstrate that GW501516 has an anti-inflammatory effect in renal tubular cells and may serve as a therapeutic candidate to attenuate tubulointerstitial lesions in proteinuric kidney diseases.
Conflict of interest statement
Figures




References
-
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. - PubMed
-
- Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis. 1996;27:765–775. - PubMed
-
- Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol. 1993;13:385–398. - PubMed
-
- Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002;62:1628–1637. - PubMed
-
- Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, et al. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int. 1998;53:1608–1615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

