Angiopoietin-like 4 regulates epidermal differentiation
- PMID: 21966511
- PMCID: PMC3178651
- DOI: 10.1371/journal.pone.0025377
Angiopoietin-like 4 regulates epidermal differentiation
Abstract
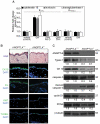
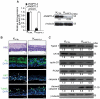
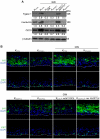
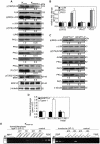
The nuclear hormone receptor PPARβ/δ is integral to efficient wound re-epithelialization and implicated in epidermal maturation. However, the mechanism underlying the latter process of epidermal differentiation remains unclear. We showed that ligand-activated PPARβ/δ indirectly stimulated keratinocyte differentiation, requiring de novo gene transcription and protein translation. Using organotypic skin cultures constructed from PPARβ/δ- and angiopoietin-like 4 (ANGPTL4)-knockdown human keratinocytes, we showed that the expression of ANGPTL4, a PPARβ/δ target gene, is essential for the receptor mediated epidermal differentiation. The pro-differentiation effect of PPARβ/δ agonist GW501516 was also abolished when keratinocytes were co-treated with PPARβ/δ antagonist GSK0660 and similarly in organotypic skin culture incubated with blocking ANGPTL4 monoclonal antibody targeted against the C-terminal fibrinogen-like domain. Our focused real-time PCR gene expression analysis comparing the skin biopsies from wildtype and ANGPTL4-knockout mice confirmed a consistent down-regulation of numerous genes involved in epidermal differentiation and proliferation in the ANGPTL4-knockout skin. We further showed that the deficiency of ANGPTL4 in human keratinocytes and mice skin have diminished expression of various protein kinase C isotypes and phosphorylated transcriptional factor activator protein-1, which are well-established for their roles in keratinocyte differentiation. Chromatin immunoprecipitation confirmed that ANGPTL4 stimulated the activation and binding of JUNB and c-JUN to the promoter region of human involucrin and transglutaminase type 1 genes, respectively. Taken together, we showed that PPARβ/δ regulates epidermal maturation via ANGPTL4-mediated signalling pathway.
Conflict of interest statement
Figures
Similar articles
-
Stable over-expression of PPARβ/δ and PPARγ to examine receptor signaling in human HaCaT keratinocytes.Cell Signal. 2011 Dec;23(12):2039-50. doi: 10.1016/j.cellsig.2011.07.020. Epub 2011 Aug 4. Cell Signal. 2011. PMID: 21843636 Free PMC article.
-
PPARβ/δ-ANGPTL4 axis mediates the promotion of mono-2-ethylhexyl phthalic acid on MYCN-amplified neuroblastoma development.Sci Total Environ. 2024 Feb 20;912:168949. doi: 10.1016/j.scitotenv.2023.168949. Epub 2023 Nov 30. Sci Total Environ. 2024. PMID: 38042186
-
Transcriptional profiling identifies functional interactions of TGF β and PPAR β/δ signaling: synergistic induction of ANGPTL4 transcription.J Biol Chem. 2010 Sep 17;285(38):29469-79. doi: 10.1074/jbc.M110.142018. Epub 2010 Jul 1. J Biol Chem. 2010. PMID: 20595396 Free PMC article.
-
Regulatory mechanisms mediated by peroxisome proliferator-activated receptor-β/δ in skin cancer.Mol Carcinog. 2019 Sep;58(9):1612-1622. doi: 10.1002/mc.23033. Epub 2019 May 6. Mol Carcinog. 2019. PMID: 31062422 Free PMC article. Review.
-
Harnessing the benefits of PPARβ/δ agonists.Life Sci. 2013 Dec 18;93(25-26):963-7. doi: 10.1016/j.lfs.2013.10.022. Epub 2013 Nov 1. Life Sci. 2013. PMID: 24184294 Review.
Cited by
-
Identification of a novel PPARβ/δ/miR-21-3p axis in UV-induced skin inflammation.EMBO Mol Med. 2016 Aug 1;8(8):919-36. doi: 10.15252/emmm.201505384. Print 2016 Aug. EMBO Mol Med. 2016. PMID: 27250636 Free PMC article.
-
Cytosolic and Transmembrane Protein Extraction Methods of Breast and Ovarian Cancer Cells: A Comparative Study.J Biomol Tech. 2018 Sep;29(3):71-78. doi: 10.7171/jbt.18-2903-002. J Biomol Tech. 2018. PMID: 30174558 Free PMC article.
-
ANGPTL4 regulate glutamine metabolism and fatty acid oxidation in nonsmall cell lung cancer cells.J Cell Mol Med. 2022 Apr;26(7):1876-1885. doi: 10.1111/jcmm.16879. Epub 2022 Mar 13. J Cell Mol Med. 2022. PMID: 35285130 Free PMC article.
-
The transcription factor sterile alpha motif (SAM) pointed domain-containing ETS transcription factor (SPDEF) is required for E-cadherin expression in prostate cancer cells.J Biol Chem. 2013 Apr 26;288(17):12222-31. doi: 10.1074/jbc.M112.434225. Epub 2013 Feb 28. J Biol Chem. 2013. Retraction in: J Biol Chem. 2014 Aug 8;289(32):22020. doi: 10.1074/jbc.A112.434225. PMID: 23449978 Free PMC article. Retracted.
-
Evolution of the angiopoietin-like gene family in teleosts and their role in skin regeneration.BMC Evol Biol. 2017 Jan 13;17(1):14. doi: 10.1186/s12862-016-0859-x. BMC Evol Biol. 2017. PMID: 28086749 Free PMC article.
References
-
- Dotto GP. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit Rev Oral Biol Med. 1999;10:442–457. - PubMed
-
- Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. - PubMed
-
- Eckert RL, Welter JF. Transcription factor regulation of epidermal keratinocyte gene expression. Mol Biol Rep. 1996;23:59–70. - PubMed
-
- Hanley K, Devaskar UP, Hicks SJ, Jiang Y, Crumrine D, et al. Hypothyroidism delays fetal stratum corneum development in mice. Pediatr Res. 1997;42:610–614. - PubMed
-
- Kömüves LG, Hanley K, Jiang Y, Elias PM, Williams ML, et al. Ligands and activators of nuclear hormone receptors regulate epidermal differentiation during fetal rat skin development. J Invest Dermatol. 1998;111:429–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

