Role of α(5)β(1) Integrin Up-regulation in Radiation-Induced Invasion by Human Pancreatic Cancer Cells
- PMID: 21966545
- PMCID: PMC3162303
- DOI: 10.1593/tlo.11133
Role of α(5)β(1) Integrin Up-regulation in Radiation-Induced Invasion by Human Pancreatic Cancer Cells
Abstract
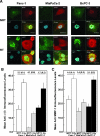
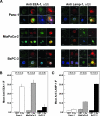
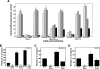
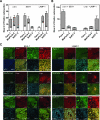
RADIOTHERAPY IS USED IN THE MANAGEMENT OF PANCREATIC CANCER BECAUSE OF ITS HIGH PROPENSITY FOR LOCOREGIONAL RELAPSE: one third of patients succumb to localized disease. Thus, strategies to improve the efficacy of radiotherapy in pancreatic cancer are important to pursue. We used naturally serum-free, selectively permeable basement membranes and confocal microscopy of fluorescent antibody-stained human Panc-1, MiaPaCa-2, and BxPC-3 pancreatic cancer cell lines to investigate the effects of ionizing radiation on α(5)β(1) integrin fibronectin receptor expression and on α(5)β(1)-mediated invasion. We report that radiation rapidly induces pancreatic cancer cell invasion, and that radiation-induced invasion is caused by up-regulation of α(5)β(1) integrin fibronectin receptors by transcriptional and/or postendocytic recycling mechanisms. We also report that radiation causes α(5)β(1) up-regulation in Panc-1, MiaPaCa-2, and BxPC-3 tumor xenografts and that upregulated α(5)β(1) colocalizes with upregulated early or late endosomes in Panc-1 or BxPC-3 tumors, respectively, although it may colocalize significantly with both endosome types in MiaPaCa-2 tumors. Our results suggest that systemic inhibition of α(5)β(1)-mediated invasion might be an effective way to reduce radiation-induced pancreatic cancer cell invasion, thereby improving the efficacy of radiotherapy.
Figures

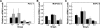
References
-
- Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11:612–623. - PubMed
-
- Chang BW, Siccion E, Saif MW. Updates in locally advanced pancreatic cancer. Highlights from the “2010 ASCO Annual Meeting”. Chicago, IL, USA June 4–8, 2010. JOP. 2010;11:313–316. - PubMed
-
- Baluna RG, Eng TY, Thomas CR. Adhesion molecules in radiotherapy. Radiat Res. 2006;166:819–831. - PubMed
-
- Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res. 2001;61:2744–2750. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
