Stepwise proteolytic activation of type I procollagen to collagen within the secretory pathway of tendon fibroblasts in situ
- PMID: 21967573
- PMCID: PMC3430002
- DOI: 10.1042/BJ20111379
Stepwise proteolytic activation of type I procollagen to collagen within the secretory pathway of tendon fibroblasts in situ
Abstract
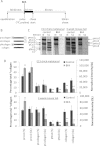
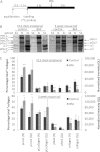
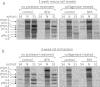
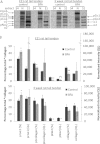
Proteolytic cleavage of procollagen I to collagen I is essential for the formation of collagen fibrils in the extracellular matrix of vertebrate tissues. Procollagen is cleaved by the procollagen N- and C-proteinases, which remove the respective N- and C-propeptides from procollagen. Procollagen processing is initiated within the secretory pathway in tendon fibroblasts, which are adept in assembling an ordered extracellular matrix of collagen fibrils in vivo. It was thought that intracellular processing was restricted to the TGN (trans-Golgi network). In the present study, brefeldin A treatment of tendon explant cultures showed that N-proteinase activity is present in the resulting fused ER (endoplasmic reticulum)-Golgi compartment, but that C-proteinase activity is restricted to the TGN in embryonic chick tendon fibroblasts. In late embryonic and postnatal rat tail and postnatal mouse tail tendon, C-proteinase activity was detected in TGN and pre-TGN compartments. Preventing activation of the procollagen N- and C-proteinases with the furin inhibitor Dec-RVKR-CMK (decanoyl-Arg-Val-Lys-Arg-chloromethylketone) indicated that only a fraction of intracellular procollagen cleavage was mediated by newly activated proteinases. In conclusion, the N-propeptides are removed earlier in the secretory pathway than the C-propeptides. The removal of the C-propeptides in post-Golgi compartments most probably indicates preparation of collagen molecules for fibril formation at the cell-matrix interface.
Figures




References
-
- Chapman J. A., Tzaphlidou M., Meek K. M., Kadler K. E. The collagen fibril: a model system for studying the staining and fixation of a protein. Electron Microsc. Rev. 1990;3:143–182. - PubMed
-
- Kadler K. E., Hojima Y., Prockop D. J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase: assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987;262:15696–15701. - PubMed
-
- Bonfanti L., Mironov A. A., Martinez-Menarguez J. A., Martella O., Fusella A., Baldassarre M., Buccione R., Geuze H. J., Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

