Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin
- PMID: 21967767
- PMCID: PMC3201198
- DOI: 10.1084/jem.20110560
Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin
Abstract
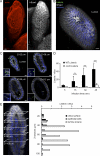
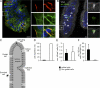
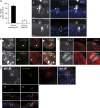
Listeria monocytogenes (Lm) is a foodborne pathogen that crosses the intestinal barrier upon interaction between its surface protein InlA and its species-specific host receptor E-cadherin (Ecad). Ecad, the key constituent of adherens junctions, is typically situated below tight junctions and therefore considered inaccessible from the intestinal lumen. In this study, we investigated how Lm specifically targets its receptor on intestinal villi and crosses the intestinal epithelium to disseminate systemically. We demonstrate that Ecad is luminally accessible around mucus-expelling goblet cells (GCs), around extruding enterocytes at the tip and lateral sides of villi, and in villus epithelial folds. We show that upon preferential adherence to accessible Ecad on GCs, Lm is internalized, rapidly transcytosed across the intestinal epithelium, and released in the lamina propria by exocytosis from where it disseminates systemically. Together, these results show that Lm exploits intrinsic tissue heterogeneity to access its receptor and reveal transcytosis as a novel and unanticipated pathway that is hijacked by Lm to breach the intestinal epithelium and cause systemic infection.
Figures




References
-
- Ameen N.A., Marino C., Salas P.J. 2003. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am. J. Physiol. Cell Physiol. 284:C429–C438 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

