Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis
- PMID: 21967897
- PMCID: PMC3208135
- DOI: 10.4049/jimmunol.1101186
Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis
Abstract
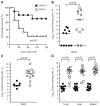
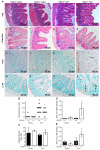
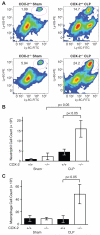
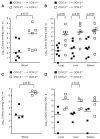
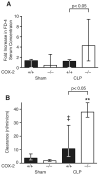
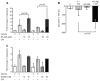
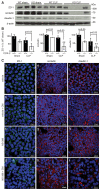
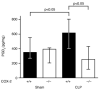
Sepsis remains the leading cause of death in critically ill patients, despite modern advances in critical care. Intestinal barrier dysfunction may lead to secondary bacterial translocation and the development of the multiple organ dysfunction syndrome during sepsis. Cyclooxygenase (COX)-2 is highly upregulated in the intestine during sepsis, and we hypothesized that it may be critical in the maintenance of intestinal epithelial barrier function during peritonitis-induced polymicrobial sepsis. COX-2(-/-) and COX-2(+/+) BALB/c mice underwent cecal ligation and puncture (CLP) or sham surgery. Mice chimeric for COX-2 were derived by bone marrow transplantation and underwent CLP. C2BBe1 cells, an intestinal epithelial cell line, were treated with the COX-2 inhibitor NS-398, PGD(2), or vehicle and stimulated with cytokines. COX-2(-/-) mice developed exaggerated bacteremia and increased mortality compared with COX-2(+/+) mice following CLP. Mice chimeric for COX-2 exhibited the recipient phenotype, suggesting that epithelial COX-2 expression in the ileum attenuates bacteremia following CLP. Absence of COX-2 significantly increased epithelial permeability of the ileum and reduced expression of the tight junction proteins zonula occludens-1, occludin, and claudin-1 in the ileum following CLP. Furthermore, PGD(2) attenuated cytokine-induced hyperpermeability and zonula occludens-1 downregulation in NS-398-treated C2BBe1 cells. Our findings reveal that absence of COX-2 is associated with enhanced intestinal epithelial permeability and leads to exaggerated bacterial translocation and increased mortality during peritonitis-induced sepsis. Taken together, our results suggest that epithelial expression of COX-2 in the ileum is a critical modulator of tight junction protein expression and intestinal barrier function during sepsis.
Figures

Similar articles
-
Low doses of celecoxib attenuate gut barrier failure during experimental peritonitis.Lab Invest. 2013 Dec;93(12):1265-75. doi: 10.1038/labinvest.2013.119. Epub 2013 Oct 14. Lab Invest. 2013. PMID: 24126890 Free PMC article.
-
Effects of intestinal alkaline phosphatase on intestinal barrier function in a cecal ligation and puncture (CLP)-induced mouse model for sepsis.Neurogastroenterol Motil. 2020 Mar;32(3):e13754. doi: 10.1111/nmo.13754. Epub 2019 Nov 21. Neurogastroenterol Motil. 2020. PMID: 31751495
-
Glucose-Insulin-Potassium Alleviates Intestinal Mucosal Barrier Injuries Involving Decreased Expression of Uncoupling Protein 2 and NLR Family-Pyrin Domain-Containing 3 Inflammasome in Polymicrobial Sepsis.Biomed Res Int. 2017;2017:4702067. doi: 10.1155/2017/4702067. Epub 2017 Mar 27. Biomed Res Int. 2017. PMID: 28428961 Free PMC article.
-
Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis.PLoS One. 2013 May 22;8(5):e62792. doi: 10.1371/journal.pone.0062792. Print 2013. PLoS One. 2013. PMID: 23717394 Free PMC article.
-
Protective effect of emodin on intestinal epithelial tight junction barrier integrity in rats with sepsis induced by cecal ligation and puncture.Exp Ther Med. 2020 Jun;19(6):3521-3530. doi: 10.3892/etm.2020.8625. Epub 2020 Mar 24. Exp Ther Med. 2020. PMID: 32346413 Free PMC article.
Cited by
-
Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators.Crit Care Med. 2016 Dec;44(12):e1236-e1245. doi: 10.1097/CCM.0000000000001999. Crit Care Med. 2016. PMID: 27513357 Free PMC article.
-
A Novel Protective Role for Matrix Metalloproteinase-8 in the Pulmonary Vasculature.Am J Respir Crit Care Med. 2021 Dec 15;204(12):1433-1451. doi: 10.1164/rccm.202108-1863OC. Am J Respir Crit Care Med. 2021. PMID: 34550870 Free PMC article.
-
The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness.Crit Care Clin. 2016 Apr;32(2):203-12. doi: 10.1016/j.ccc.2015.11.004. Epub 2016 Feb 4. Crit Care Clin. 2016. PMID: 27016162 Free PMC article. Review.
-
MCL1 Is Required for Maintenance of Intestinal Homeostasis and Prevention of Carcinogenesis in Mice.Gastroenterology. 2020 Jul;159(1):183-199. doi: 10.1053/j.gastro.2020.03.017. Epub 2020 Mar 14. Gastroenterology. 2020. PMID: 32179094 Free PMC article.
-
Diclofenac causes more leakage than naproxen in anastomoses in the small intestine of the rat.Int J Colorectal Dis. 2013 Sep;28(9):1209-16. doi: 10.1007/s00384-013-1652-6. Epub 2013 Feb 10. Int J Colorectal Dis. 2013. PMID: 23397591
References
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. - PubMed
-
- Anaya DA, Nathens AB. Risk factors for severe sepsis in secondary peritonitis. Surg. Infect. 2003;4:355–362. - PubMed
-
- Klein T, Shephard P, Kleinert H, Komhoff M. Regulation of cyclooxygenase-2 expression by cyclic AMP. Biochim. Biophys. Acta. 2007;1773:1605–1618. - PubMed
-
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088199/DK/NIDDK NIH HHS/United States
- K08 GM083207/GM/NIGMS NIH HHS/United States
- R01 DK051362/DK/NIDDK NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- R01 GM053249/GM/NIGMS NIH HHS/United States
- U01 AI061246/AI/NIAID NIH HHS/United States
- R01 AI061246/AI/NIAID NIH HHS/United States
- R01 HL060788/HL/NHLBI NIH HHS/United States
- T34 GM087193/GM/NIGMS NIH HHS/United States
- L30 GM089116/GM/NIGMS NIH HHS/United States
- P01 HL108801/HL/NHLBI NIH HHS/United States
- R37 DK044319/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

