Congenital hyperinsulinism: current trends in diagnosis and therapy
- PMID: 21967988
- PMCID: PMC3199232
- DOI: 10.1186/1750-1172-6-63
Congenital hyperinsulinism: current trends in diagnosis and therapy
Abstract
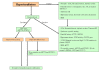
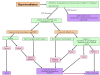
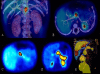
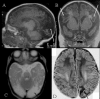
Congenital hyperinsulinism (HI) is an inappropriate insulin secretion by the pancreatic β-cells secondary to various genetic disorders. The incidence is estimated at 1/50, 000 live births, but it may be as high as 1/2, 500 in countries with substantial consanguinity. Recurrent episodes of hyperinsulinemic hypoglycemia may expose to high risk of brain damage. Hypoglycemias are diagnosed because of seizures, a faint, or any other neurological symptom, in the neonatal period or later, usually within the first two years of life. After the neonatal period, the patient can present the typical clinical features of a hypoglycemia: pallor, sweat and tachycardia. HI is a heterogeneous disorder with two main clinically indistinguishable histopathological lesions: diffuse and focal. Atypical lesions are under characterization. Recessive ABCC8 mutations (encoding SUR1, subunit of a potassium channel) and, more rarely, recessive KCNJ11 (encoding Kir6.2, subunit of the same potassium channel) mutations, are responsible for most severe diazoxide-unresponsive HI. Focal HI, also diazoxide-unresponsive, is due to the combination of a paternally-inherited ABCC8 or KCNJ11 mutation and a paternal isodisomy of the 11p15 region, which is specific to the islets cells within the focal lesion. Genetics and 18F-fluoro-L-DOPA positron emission tomography (PET) help to diagnose diffuse or focal forms of HI. Hypoglycemias must be rapidly and intensively treated to prevent severe and irreversible brain damage. This includes a glucose load and/or a glucagon injection, at the time of hypoglycemia, to correct it. Then a treatment to prevent the recurrence of hypoglycemia must be set, which may include frequent and glucose-enriched feeding, diazoxide and octreotide. When medical and dietary therapies are ineffective, or when a focal HI is suspected, surgical treatment is required. Focal HI may be definitively cured when the partial pancreatectomy removes the whole lesion. By contrast, the long-term outcome of diffuse HI after subtotal pancreatectomy is characterized by a high risk of diabetes, but the time of its onset is hardly predictable.
Figures


References
-
- Bruining GJ. Recent advances in hyperinsulinism and the pathogenesis of diabetes mellitus. Current Opinion in Pediatrics. 1990;2:758–765. doi: 10.1097/00008480-199008000-00024. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

