Plasma drug activity assay for treatment optimization in tuberculosis patients
- PMID: 21968363
- PMCID: PMC3232779
- DOI: 10.1128/AAC.05561-11
Plasma drug activity assay for treatment optimization in tuberculosis patients
Abstract
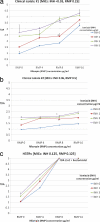
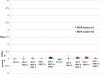
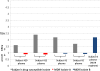
Low antituberculosis (TB) drug levels are common, but their clinical significance remains unclear, and methods of measurement are resource intensive. Subjects initiating treatment for sputum smear-positive pulmonary TB were enrolled from Kibong'oto National TB Hospital, Tanzania, and levels of isoniazid, rifampin, ethambutol, and pyrazinamide were measured at the time of typical peak plasma concentration (C(2 h)). To evaluate the significance of the effect of observed drug levels on Mycobacterium tuberculosis growth, a plasma TB drug activity (TDA) assay was developed using the Bactec MGIT system. Time to detection of plasma-cocultured M. tuberculosis versus time to detection of control growth was defined as a TDA ratio. TDA assays were later performed using the subject's own M. tuberculosis isolate and C(2 h) plasma from the Tanzanian cohort and compared to drug levels and clinical outcomes. Sixteen subjects with a mean age of 37.8 years ± 10.7 were enrolled. Fourteen (88%) had C(2 h) rifampin levels and 11 (69%) had isoniazid levels below 90% of the lower limit of the expected range. Plasma spiked with various concentrations of antituberculosis medications found TDA assay results to be unaffected by ethambutol or pyrazinamide. Yet with a range of isoniazid and rifampin concentrations, TDA exhibited a statistically significant correlation with drug level and drug MIC, and a TDA of ~1.0 indicated the presence of multidrug-resistant TB. In Tanzania, low (≤ 2.0) TDA was significantly associated with both lower isoniazid and rifampin C(2 h) levels, and very low (≤ 1.5) TDA corresponded to a trend toward lack of cure. Study of TDA compared to additional clinical outcomes and as a therapeutic management tool is warranted.
Figures

References
-
- Caminero J. A. 2006. Treatment of multidrug-resistant tuberculosis: evidence and controversies. Int. J. Tuberc. Lung Dis. 10:829–837 - PubMed
-
- Chang K. C., et al. 2008. Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur. J. Clin. Microbiol. Infect. Dis. 27:467–472 - PubMed
-
- Diacon A. H., et al. 2010. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur. J. Clin. Microbiol. Infect. Dis. 29:1561–1565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

