Polyethylenimine is a strong inhibitor of human papillomavirus and cytomegalovirus infection
- PMID: 21968369
- PMCID: PMC3256013
- DOI: 10.1128/AAC.05147-11
Polyethylenimine is a strong inhibitor of human papillomavirus and cytomegalovirus infection
Abstract
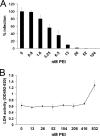
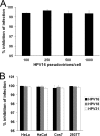
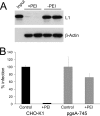
Polyethylenimines are cationic polymers with potential as delivery vectors in gene therapy and with proven antimicrobial activity. However, the antiviral activity of polyethylenimines has not been addressed in detail thus far. We have studied the inhibitory effects of a linear 25-kDa polyethylenimine on infections with human papillomaviruses and human cytomegaloviruses. Preincubation of cells with polyethylenimine blocked primary attachment of both viruses to cells, resulting in a significant reduction of infection. In addition, the dissemination of human cytomegalovirus in culture cells was efficiently reduced by recurrent administration of polyethylenimine. Polyethylenimine concentrations required for inhibition of human papillomavirus and cytomegalovirus did not cause any cytotoxic effects. Polyethylenimines and their derivatives may thus be attractive molecules for the development of antiviral microbicides.
Figures




Similar articles
-
[Influence of membrane-active polyanions on various stages of the human cytomegalovirus life cycle in vitro].Antibiot Khimioter. 2008;53(11-12):3-10. Antibiot Khimioter. 2008. PMID: 19441649 Russian.
-
Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate.Antiviral Res. 2010 Mar;85(3):532-40. doi: 10.1016/j.antiviral.2010.01.003. Epub 2010 Jan 18. Antiviral Res. 2010. PMID: 20083141
-
In vitro and in vivo activity of 1-O-hexadecylpropanediol-3-phospho-ganciclovir and 1-O-hexadecylpropanediol-3-phospho-penciclovir in cytomegalovirus and herpes simplex virus infections.Antivir Chem Chemother. 2001 Jan;12(1):61-70. doi: 10.1177/095632020101200104. Antivir Chem Chemother. 2001. PMID: 11437323
-
Value of animal models to evaluate agents with potential activity against human cytomegalovirus.Transplant Proc. 1991 Jun;23(3 Suppl 3):152-5, discussion 155. Transplant Proc. 1991. PMID: 1648821 Review. No abstract available.
-
HPV proteins as targets for therapeutic intervention.Antivir Ther. 2004 Oct;9(5):665-78. Antivir Ther. 2004. PMID: 15535404 Review.
Cited by
-
Linear biocompatible glyco-polyamidoamines as dual action mode virus infection inhibitors with potential as broad-spectrum microbicides for sexually transmitted diseases.Sci Rep. 2016 Sep 19;6:33393. doi: 10.1038/srep33393. Sci Rep. 2016. PMID: 27641362 Free PMC article.
-
Protamine Sulfate Is a Potent Inhibitor of Human Papillomavirus Infection In Vitro and In Vivo.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0151321. doi: 10.1128/AAC.01513-21. Epub 2021 Nov 1. Antimicrob Agents Chemother. 2022. PMID: 34723633 Free PMC article.
-
The extracellular δ-domain is essential for the formation of CD81 tetraspanin webs.Biophys J. 2014 Jul 1;107(1):100-13. doi: 10.1016/j.bpj.2014.05.028. Biophys J. 2014. PMID: 24988345 Free PMC article.
-
Antiviral Polymer Brushes by Visible-Light-Induced, Oxygen-Tolerant Covalent Surface Coating.ACS Omega. 2022 Oct 20;7(43):38371-38379. doi: 10.1021/acsomega.2c03214. eCollection 2022 Nov 1. ACS Omega. 2022. PMID: 36340175 Free PMC article.
-
Self-assembled cationic amphiphiles as antimicrobial peptides mimics: Role of hydrophobicity, linkage type, and assembly state.Nanomedicine. 2017 Feb;13(2):343-352. doi: 10.1016/j.nano.2016.07.018. Epub 2016 Aug 9. Nanomedicine. 2017. PMID: 27520722 Free PMC article.
References
-
- Andreoni M, Faircloth M, Vugler L, Britt WJ. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157–167 - PubMed
-
- Azzam T, Domb AJ. 2004. Current developments in gene transfection agents. Curr. Drug Deliv. 1:165–193 - PubMed
-
- Baba M, Snoeck R, Pauwels R, de Clercq E. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742–1745 - PMC - PubMed
-
- Bernfield M, et al. 1992. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8:365–393 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

