Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association
- PMID: 21969010
- PMCID: PMC3719404
- DOI: 10.1161/CIR.0b013e3182340239
Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association
Abstract
Background and purpose: The guidelines presented in this consensus statement are intended to serve researchers, clinicians, reviewers, and regulators in the selection of the most appropriate primary outcome for a clinical trial of cardiac arrest therapies. The American Heart Association guidelines for the treatment of cardiac arrest depend on high-quality clinical trials, which depend on the selection of a meaningful primary outcome. Because this selection process has been the subject of much controversy, a consensus conference was convened with national and international experts, the National Institutes of Health, and the US Food and Drug Administration.
Methods: The Research Working Group of the American Heart Association Emergency Cardiovascular Care Committee nominated subject leaders, conference attendees, and writing group members on the basis of their expertise in clinical trials and a diverse perspective of cardiovascular and neurological outcomes (see the online-only Data Supplement). Approval was obtained from the Emergency Cardiovascular Care Committee and the American Heart Association Manuscript Oversight Committee. Preconference position papers were circulated for review; the conference was held; and postconference consensus documents were circulated for review and comments were invited from experts, conference attendees, and writing group members. Discussions focused on (1) when after cardiac arrest the measurement time point should occur; (2) what cardiovascular, neurological, and other physiology should be assessed; and (3) the costs associated with various end points. The final document underwent extensive revision and peer review by the Emergency Cardiovascular Care Committee, the American Heart Association Science Advisory and Coordinating Committee, and oversight committees.
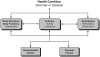
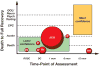
Results: There was consensus that no single primary outcome is appropriate for all studies of cardiac arrest. The best outcome measure is the pairing of a time point and physiological condition that will best answer the question under study. Conference participants were asked to assign an outcome to each of 4 hypothetical cases; however, there was not complete agreement on an ideal outcome measure even after extensive discussion and debate. There was general consensus that it is appropriate for earlier studies to enroll fewer patients and to use earlier time points such as return of spontaneous circulation, simple "alive versus dead," hospital mortality, or a hemodynamic parameter. For larger studies, a longer time point after arrest should be considered because neurological assessments fluctuate for at least 90 days after arrest. For large trials designed to have a major impact on public health policy, longer-term end points such as 90 days coupled with neurocognitive and quality-of-life assessments should be considered, as should the additional costs of this approach. For studies that will require regulatory oversight, early discussions with regulatory agencies are strongly advised. For neurological assessment of post-cardiac arrest patients, researchers may wish to use the Cerebral Performance Categories or modified Rankin Scale for global outcomes.
Conclusions: Although there is no single recommended outcome measure for trials of cardiac arrest care, the simple Cerebral Performance Categories or modified Rankin Scale after 90 days provides a reasonable outcome parameter for many trials. The lack of an easy-to-administer neurological functional outcome measure that is well validated in post-cardiac arrest patients is a major limitation to the field and should be a high priority for future development.
Conflict of interest statement
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
Figures
References
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J on behalf of American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. - PMC - PubMed
-
- Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O'Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S640–S656. - PubMed
-
- Stiell IG, Nesbitt LP, Nichol G, Maloney J, Dreyer J, Beaudoin T, Blackburn J, Wells GA OPALS Study Group. Comparison of the Cerebral Performance Category score and the Health Utilities Index for survivors of cardiac arrest. Ann Emerg Med. 2009;53:241–248. - PubMed
-
- Nichol G, Powell J, van Ottingham L, Maier R, Rea T, Christenson J, Hallstrom A. Consent in resuscitation trials: benefit or harm for patients and society? Resuscitation. 2006;70:360–368. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

