Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth
- PMID: 21969134
- PMCID: PMC3268013
- DOI: 10.1007/s10456-011-9236-y
Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth
Abstract
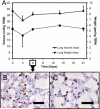
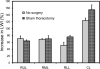
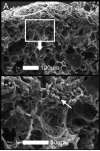
Growth of the remaining lung after pneumonectomy has been observed in many mammalian species; nonetheless, the pattern and morphology of alveolar angiogenesis during compensatory growth is unknown. Here, we investigated alveolar angiogenesis in a murine model of post-pneumonectomy lung growth. As expected, the volume and weight of the remaining lung returned to near-baseline levels within 21 days of pneumonectomy. The percentage increase in lobar weight was greatest in the cardiac lobe (P < 0.001). Cell cycle flow cytometry demonstrated a peak of lung cell proliferation (12.02 ± 1.48%) 6 days after pneumonectomy. Spatial autocorrelation analysis of the cardiac lobe demonstrated clustering of similar vascular densities (positive autocorrelation) that consistently mapped to subpleural regions of the cardiac lobe. Immunohistochemical staining demonstrated increased cell density and enhanced expression of angiogenesis-related factors VEGFA, and GLUT1 in these subpleural regions. Corrosion casting and scanning electron microscopy 3-6 days after pneumonectomy demonstrated subpleural vessels with angiogenic sprouts. The monopodial sprouts appeared to be randomly oriented along the vessel axis with interbranch distances of 11.4 ± 4.8 μm in the regions of active angiogenesis. Also present within the regions of increased vascular density were frequent "holes" or "pillars" consistent with active intussusceptive angiogenesis. The mean pillar diameter was 4.2 ± 3.8 μm, and the pillars were observed in all regions of active angiogenesis. These findings indicate that the process of alveolar construction involves discrete regions of regenerative growth, particularly in the subpleural regions of the cardiac lobe, characterized by both sprouting and intussusceptive angiogenesis.
Figures




References
-
- Tatar-Kiss S, Bardocz S, Kertai P. Changes in L-ornithine decarboxylase activity in regenerating lung lobes. FEBS Lett. 1984;175:131–134. - PubMed
-
- Heuer GJ, Dunn GR. Experimental pneumectomy. Bull Johns Hopkins Hosp. 1920;31:31–42.
-
- Bremer JL. The fate of the remaining lung tissue after lobectomy or pneumonectomy. J.Thorac.Surg. 1936;6:336–343.
-
- Sery Z, Keprt E, Obrucnik M. Morphometric analysis of late adaptation of residual lung following pneumonectomy in young and adult rabbits. J. Thorac. Cardiovasc. Surg. 1969;57:549–557. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

