Exosome/microvesicle-mediated epigenetic reprogramming of cells
- PMID: 21969178
- PMCID: PMC3180104
Exosome/microvesicle-mediated epigenetic reprogramming of cells
Abstract
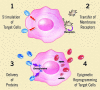
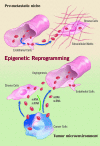
Microvesicles (MVs) are released by different cell types and may remain in the extracellular space in proximity of the cell of origin or may enter the biological fluids. MVs released by tumor cells are detectable in patients with cancer and their number in the circulation correlates with poor prognosis. Recent studies demonstrated that MVs may act as mediator of cell-to-cell communication thus ensuring short- and long-range exchange of information. Due to their pleyotropic effects, MVs may play a role in the prothrombotic state associated with cancer as well as in cancer development and progression. It has been recently shown that MVs may induce epigenetic changes in target cells by transferring genetic information. This finding suggests that tumor and stromal cells may talk each other via MVs to establish a favorable tumor niche and to promote tumor growth, invasiveness and progression. Moreover, MVs contain genetic material under the form of mRNA and microRNA, that may allow an easy screening for cancer genetic markers and offer new diagnostic and prognostic information. This review presents an overview of the many biological actions of MVs and of the potential role of MV-mediated exchange of genetic information among cells in tumor biology.
Figures

References
-
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. - PubMed
-
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. - PubMed
-
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alphagranules. Blood. 1999;94:3791–3799. - PubMed
-
- Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
