A placenta growth factor 2 variant acts as dominant negative of vascular endothelial growth factor A by heterodimerization mechanism
- PMID: 21969185
- PMCID: PMC3180054
A placenta growth factor 2 variant acts as dominant negative of vascular endothelial growth factor A by heterodimerization mechanism
Abstract
Angiogenesis is one of the crucial events for cancer development and growth and vascular endothelial growth factor (VEGF) family plays an essential role in this biological phenomenon. The members of VEGF family mainly involved in angiogenesis are VEGF-A, VEGF-B and placental growth factor (PlGF), which exert their activity through the binding and activation of two VEGF receptors, VEGFR-1 and VEGFR-2. Human VEGF-A and PlGF are expressed in different isoforms and have the peculiarity to form heterodimer if co-expressed in the same cell. The difference of two main human PlGF isoforms, PlGF1 and PlGF2, consist in the exclusive ability of PlGF2 to bind heparin and Neuropilin receptors. As previously reported for PlGF1 isoform, we have generated a PlGF2 variant named PlGF2 -DE, in which the residues D(72) and E(73) were substituted with alanine, that is unable to bind and activate VEGFR-1 but is still able to heterodimerize with VEGF. Here we report that overexpression in VEGF-A producing human tumor cell line derived from ovarian carcinoma (A2780) of PlGF2-DE variant by stable transfection, significantly reduces the production of VEGF-A homodimer via heterodimerization, determining a strong inhibition of xenograft tumor growth and associated neoangiogenesis, as well as significant reduction of monocyte-macrophage infiltration. Conversely, the overexpression of PlGF2wt, also reducing the VEGF-A homodimer production comparably to PlGF2-DE variant through the generation of VEGF-A/PlGF2 heterodimer, does not inhibit tumor growth and vessel density compared to control, but induces increase of monocyte-macrophage infiltration. Interestingly the comparison of PlGF2wt with PlGF1wt overexpression evidences a significant reduction of monocyte-macrophages recruitment as unique difference among the activity of the two PlGFwt isoforms. Therefore, the 'less soluble' PlGF2 shows a limited potential in monocyte-macrophages recruitment. In conclusion data here reported demonstrate that PlGF-DE variant acts as 'dominant negative' of VEGF-A independently from the PlGF isoform utilized, that the expression of active PlGF2 homodimer and VEGF-A/PlGF2 heterodimer is sufficient to rescue pro-angiogenic activity lost for reduction of VEGF-A due to heterodimerization mechanism, and that PlGF2 shows lower activity into recruitment of monocyte-macrophage cells compared to PlGF1 isoform.
Figures
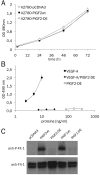
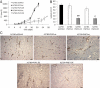

References
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. - PubMed
-
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. - PubMed
-
- Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. - PubMed
-
- Grunewald FS, Prota AE, Giese A, Ballmer-Hofer K. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta. 2010;1804:567–580. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
