Biomarkers for drug-induced renal damage and nephrotoxicity-an overview for applied toxicology
- PMID: 21969220
- PMCID: PMC3231866
- DOI: 10.1208/s12248-011-9301-x
Biomarkers for drug-induced renal damage and nephrotoxicity-an overview for applied toxicology
Abstract
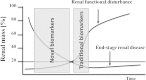
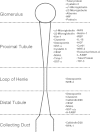
The detection of acute kidney injury (AKI) and the monitoring of chronic kidney disease (CKD) is becoming more important in industrialized countries. Because of the direct relation of kidney damage to the increasing age of the population, as well as the connection to other diseases like diabetes mellitus and congestive heart failure, renal diseases/failure has increased in the last decades. In addition, drug-induced kidney injury, especially of patients in intensive care units, is very often a cause of AKI. The need for diagnostic tools to identify drug-induced nephrotoxicity has been emphasized by the ICH-regulated agencies. This has lead to multiple national and international projects focusing on the identification of novel biomarkers to enhance drug development. Several parameters related to AKI or CKD are known and have been used for several decades. Most of these markers deliver information only when renal damage is well established, as is the case for serum creatinine. The field of molecular toxicology has spawned new options of the detection of nephrotoxicity. These new developments lead to the identification of urinary protein biomarkers, including Kim-1, clusterin, osteopontin or RPA-1, and other transcriptional biomarkers which enable the earlier detection of AKI and deliver further information about the area of nephron damage or the underlying mechanism. These biomarkers were mainly identified and qualified in rat but also for humans, several biomarkers have been described and now have to be validated. This review will give an overview of traditional and novel tools for the detection of renal damage.
Figures

References
-
- Frost & Sullivan (2007) Rang, HP (eds). Drug discovery and development. Churchill Livingstone: Elsevier
-
- Devarajan P. Novel biomarkers for the early prediction of acute kidney injury. Cancer Therapy. 2005;3:477–488.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

