The significance of G protein-coupled receptor crystallography for drug discovery
- PMID: 21969326
- PMCID: PMC3186081
- DOI: 10.1124/pr.110.003350
The significance of G protein-coupled receptor crystallography for drug discovery
Abstract
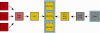
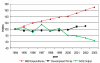
Crucial as molecular sensors for many vital physiological processes, seven-transmembrane domain G protein-coupled receptors (GPCRs) comprise the largest family of proteins targeted by drug discovery. Together with structures of the prototypical GPCR rhodopsin, solved structures of other liganded GPCRs promise to provide insights into the structural basis of the superfamily's biochemical functions and assist in the development of new therapeutic modalities and drugs. One of the greatest technical and theoretical challenges to elucidating and exploiting structure-function relationships in these systems is the emerging concept of GPCR conformational flexibility and its cause-effect relationship for receptor-receptor and receptor-effector interactions. Such conformational changes can be subtle and triggered by relatively small binding energy effects, leading to full or partial efficacy in the activation or inactivation of the receptor system at large. Pharmacological dogma generally dictates that these changes manifest themselves through kinetic modulation of the receptor's G protein partners. Atomic resolution information derived from increasingly available receptor structures provides an entrée to the understanding of these events and practically applying it to drug design. Supported by structure-activity relationship information arising from empirical screening, a unified structural model of GPCR activation/inactivation promises to both accelerate drug discovery in this field and improve our fundamental understanding of structure-based drug design in general. This review discusses fundamental problems that persist in drug design and GPCR structural determination.
Figures

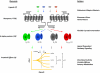

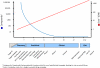



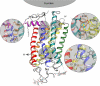
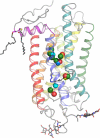
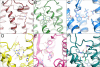
References
-
- Abola E, Kuhn P, Earnest T, Stevens RC. (2000) Automation of X-ray crystallography. Nat Struct Biol 7:973–977 - PubMed
-
- Adams PD, Grosse-Kunstleve RW. (2000) Recent developments in software for the automation of crystallographic macromolecular structure determination. Curr Opin Struct Biol 10:564–568 - PubMed
-
- Alewijnse AE, Timmerman H, Jacobs EH, Smit MJ, Roovers E, Cotecchia S, Leurs R. (2000) The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol Pharmacol 57:890–898 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

