α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson's disease
- PMID: 21969327
- PMCID: PMC3186078
- DOI: 10.1124/pr.110.003269
α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson's disease
Abstract
Parkinson's disease is a debilitating movement disorder characterized by a generalized dysfunction of the nervous system, with a particularly prominent decline in the nigrostriatal dopaminergic pathway. Although there is currently no cure, drugs targeting the dopaminergic system provide major symptomatic relief. As well, agents directed to other neurotransmitter systems are of therapeutic benefit. Such drugs may act by directly improving functional deficits in these other systems, or they may restore aberrant motor activity that arises as a result of a dopaminergic imbalance. Recent research attention has focused on a role for drugs targeting the nicotinic cholinergic systems. The rationale for such work stems from basic research findings that there is an extensive overlap in the organization and function of the nicotinic cholinergic and dopaminergic systems in the basal ganglia. In addition, nicotinic acetylcholine receptor (nAChR) drugs could have clinical potential for Parkinson's disease. Evidence for this proposition stems from studies with experimental animal models showing that nicotine protects against neurotoxin-induced nigrostriatal damage and improves motor complications associated with l-DOPA, the "gold standard" for Parkinson's disease treatment. Nicotine interacts with multiple central nervous system receptors to generate therapeutic responses but also produces side effects. It is important therefore to identify the nAChR subtypes most beneficial for treating Parkinson's disease. Here we review nAChRs with particular emphasis on the subtypes that contribute to basal ganglia function. Accumulating evidence suggests that drugs targeting α6β2* and α4β2* nAChR may prove useful in the management of Parkinson's disease.
Figures
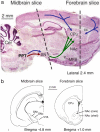
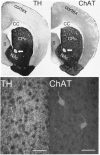
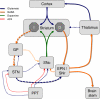
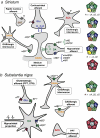


Similar articles
-
Multiple roles for nicotine in Parkinson's disease.Biochem Pharmacol. 2009 Oct 1;78(7):677-85. doi: 10.1016/j.bcp.2009.05.003. Epub 2009 May 9. Biochem Pharmacol. 2009. PMID: 19433069 Free PMC article. Review.
-
Nicotinic receptors as CNS targets for Parkinson's disease.Biochem Pharmacol. 2007 Oct 15;74(8):1224-34. doi: 10.1016/j.bcp.2007.06.015. Epub 2007 Jun 17. Biochem Pharmacol. 2007. PMID: 17631864 Free PMC article. Review.
-
Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice.Biochem Pharmacol. 2013 Oct 15;86(8):1153-62. doi: 10.1016/j.bcp.2013.06.027. Epub 2013 Jul 4. Biochem Pharmacol. 2013. PMID: 23831952 Free PMC article.
-
α6ß2* and α4ß2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson's disease.Mol Pharmacol. 2010 Nov;78(5):971-80. doi: 10.1124/mol.110.067561. Epub 2010 Aug 23. Mol Pharmacol. 2010. PMID: 20732972 Free PMC article.
-
Characterization of AN6001, a positive allosteric modulator of α6β2-containing nicotinic acetylcholine receptors.Biochem Pharmacol. 2020 Apr;174:113788. doi: 10.1016/j.bcp.2019.113788. Epub 2019 Dec 27. Biochem Pharmacol. 2020. PMID: 31887290
Cited by
-
Human α4β2 nicotinic acetylcholine receptor as a novel target of oligomeric α-synuclein.PLoS One. 2013;8(2):e55886. doi: 10.1371/journal.pone.0055886. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437071 Free PMC article.
-
Nicotinic Receptor Intervention in Parkinson's Disease: Future Directions.Clin Pharmacol Transl Med. 2017;1(1):14-19. Epub 2017 Mar 6. Clin Pharmacol Transl Med. 2017. PMID: 29863173 Free PMC article.
-
Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson's disease.J Pharmacol Exp Ther. 2013 Oct;347(1):225-34. doi: 10.1124/jpet.113.207639. Epub 2013 Jul 31. J Pharmacol Exp Ther. 2013. PMID: 23902940 Free PMC article.
-
pKa determination of histidine residues in α-conotoxin MII peptides by 1H NMR and constant pH molecular dynamics simulation.J Phys Chem B. 2013 Mar 7;117(9):2653-61. doi: 10.1021/jp3117227. Epub 2013 Feb 25. J Phys Chem B. 2013. PMID: 23336579 Free PMC article.
-
Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson's disease.Front Aging Neurosci. 2015 Jan 9;6:340. doi: 10.3389/fnagi.2014.00340. eCollection 2014. Front Aging Neurosci. 2015. PMID: 25620929 Free PMC article. Review.
References
-
- Ahlskog JE, Muenter MD. (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458 - PubMed
-
- Albin RL, Young AB, Penney JB. (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375 - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. (1997) Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9:2734–2742 - PubMed
-
- Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernández-Crehuet Navajas R. (2004) Smoking and Parkinson's disease: systematic review of prospective studies. Mov Disord 19:614–621 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

