Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK)
- PMID: 21969364
- PMCID: PMC3220470
- DOI: 10.1074/jbc.M111.279307
Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK)
Abstract
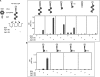
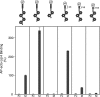
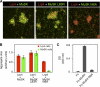
Neuromuscular synapse formation depends upon coordinated interactions between motor neurons and muscle fibers, leading to the formation of a highly specialized postsynaptic membrane and a highly differentiated nerve terminal. Synapse formation begins as motor axons approach muscles that are prepatterned in the prospective synaptic region in a manner that depends upon Lrp4, a member of the LDL receptor family, and muscle-specific kinase (MuSK), a receptor tyrosine kinase. Motor axons supply Agrin, which binds Lrp4 and stimulates further MuSK phosphorylation, stabilizing nascent synapses. How Agrin binds Lrp4 and stimulates MuSK kinase activity is poorly understood. Here, we demonstrate that Agrin binds to the N-terminal region of Lrp4, including a subset of the LDLa repeats and the first of four β-propeller domains, which promotes association between Lrp4 and MuSK and stimulates MuSK kinase activity. In addition, we show that Agrin stimulates the formation of a functional complex between Lrp4 and MuSK on the surface of myotubes in the absence of the transmembrane and intracellular domains of Lrp4. Further, we demonstrate that the first Ig-like domain in MuSK, which shares homology with the NGF-binding region in Tropomyosin Receptor Kinase (TrKA), is required for MuSK to bind Lrp4. These findings suggest that Lrp4 is a cis-acting ligand for MuSK, whereas Agrin functions as an allosteric and paracrine regulator to promote association between Lrp4 and MuSK.
Figures



References
-
- Sanes J. R., Lichtman J. W. (2001) Nat. Rev. Neurosci. 2, 791–805 - PubMed
-
- Burden S. J. (2011) Cell 144, 826–826.e1 - PubMed
-
- Okada K., Inoue A., Okada M., Murata Y., Kakuta S., Jigami T., Kubo S., Shiraishi H., Eguchi K., Motomura M., Akiyama T., Iwakura Y., Higuchi O., Yamanashi Y. (2006) Science 312, 1802–1805 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

