Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial
- PMID: 21969517
- PMCID: PMC3565012
- DOI: 10.1200/JCO.2011.36.5742
Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial
Abstract
Purpose: The trial objectives were to identify the maximum-tolerated dose (MTD) of first-line gemcitabine plus nab-paclitaxel in metastatic pancreatic adenocarcinoma and to provide efficacy and safety data. Additional objectives were to evaluate positron emission tomography (PET) scan response, secreted protein acidic and rich in cysteine (SPARC), and CA19-9 levels in relation to efficacy. Subsequent preclinical studies investigated the changes involving the pancreatic stroma and drug uptake.
Patients and methods: Patients with previously untreated advanced pancreatic cancer were treated with 100, 125, or 150 mg/m(2) nab-paclitaxel followed by gemcitabine 1,000 mg/m(2) on days 1, 8, and 15 every 28 days. In the preclinical study, mice were implanted with human pancreatic cancers and treated with study agents.
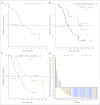
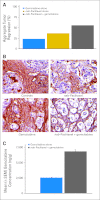
Results: A total of 20, 44, and three patients received nab-paclitaxel at 100, 125, and 150 mg/m(2), respectively. The MTD was 1,000 mg/m(2) of gemcitabine plus 125 mg/m(2) of nab-paclitaxel once a week for 3 weeks, every 28 days. Dose-limiting toxicities were sepsis and neutropenia. At the MTD, the response rate was 48%, with 12.2 median months of overall survival (OS) and 48% 1-year survival. Improved OS was observed in patients who had a complete metabolic response on [(18)F]fluorodeoxyglucose PET. Decreases in CA19-9 levels were correlated with increased response rate, progression-free survival, and OS. SPARC in the stroma, but not in the tumor, was correlated with improved survival. In mice with human pancreatic cancer xenografts, nab-paclitaxel alone and in combination with gemcitabine depleted the desmoplastic stroma. The intratumoral concentration of gemcitabine was increased by 2.8-fold in mice receiving nab-paclitaxel plus gemcitabine versus those receiving gemcitabine alone.
Conclusion: The regimen of nab-paclitaxel plus gemcitabine has tolerable adverse effects with substantial antitumor activity, warranting phase III evaluation.
Trial registration: ClinicalTrials.gov NCT00398086.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- American Cancer Society. Cancer facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. p. 65.
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. - PubMed
-
- Eli Lilly. Gemzar prescribing information. Indianapolis, IN: 1996. pp. 1–23.
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

