Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions
- PMID: 21969533
- PMCID: PMC3193208
- DOI: 10.1073/pnas.1108795108
Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions
Abstract
Cell-penetrating peptides (CPPs), such as the HIV TAT peptide, are able to translocate across cellular membranes efficiently. A number of mechanisms, from direct entry to various endocytotic mechanisms (both receptor independent and receptor dependent), have been observed but how these specific amino acid sequences accomplish these effects is unknown. We show how CPP sequences can multiplex interactions with the membrane, the actin cytoskeleton, and cell-surface receptors to facilitate different translocation pathways under different conditions. Using "nunchuck" CPPs, we demonstrate that CPPs permeabilize membranes by generating topologically active saddle-splay ("negative Gaussian") membrane curvature through multidentate hydrogen bonding of lipid head groups. This requirement for negative Gaussian curvature constrains but underdetermines the amino acid content of CPPs. We observe that in most CPP sequences decreasing arginine content is offset by a simultaneous increase in lysine and hydrophobic content. Moreover, by densely organizing cationic residues while satisfying the above constraint, TAT peptide is able to combine cytoskeletal remodeling activity with membrane translocation activity. We show that the TAT peptide can induce structural changes reminiscent of macropinocytosis in actin-encapsulated giant vesicles without receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

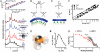
 , characteristic of a bicontinuous cubic Pn3m phase (h, k, l are Miller indices; a is the lattice parameter). Tetraarginine blocks connected with a short PEG spacer (R4-(PEG)5-R4) induces a Pn3m phase (P/L = 1/40) with a larger lattice constant. SAXS data for R4-(PEG)27-R4, with a longer PEG spacer, show that the Pn3m phase is suppressed, replaced by HII and Lα phases with no saddle-splay curvature (P/L = 1/40). (B) Multidentate coordination of arginine’s guanidinium side chain induces positive curvature strain along the peptide. (C) Monodentate coordination of lysine’s amino side chain does not induce positive curvature. (D) The cubic Pn3m phase (the zero-mean-curvature surface at the midplane between the two membrane leaflets) (E) The average induced Gaussian curvature
, characteristic of a bicontinuous cubic Pn3m phase (h, k, l are Miller indices; a is the lattice parameter). Tetraarginine blocks connected with a short PEG spacer (R4-(PEG)5-R4) induces a Pn3m phase (P/L = 1/40) with a larger lattice constant. SAXS data for R4-(PEG)27-R4, with a longer PEG spacer, show that the Pn3m phase is suppressed, replaced by HII and Lα phases with no saddle-splay curvature (P/L = 1/40). (B) Multidentate coordination of arginine’s guanidinium side chain induces positive curvature strain along the peptide. (C) Monodentate coordination of lysine’s amino side chain does not induce positive curvature. (D) The cubic Pn3m phase (the zero-mean-curvature surface at the midplane between the two membrane leaflets) (E) The average induced Gaussian curvature  ; χ = -2 and Ao = 1.919 for Pn3m) is maximized near NR = 9, where observed membrane transduction activity is empirically highest. (F) ANTP, TAT peptide, R9 all induce the cubic Pn3m phase in membranes (DOPS∶DOPE = 20∶80 at P/L = 1/40). (G) The ratio of the number of arginines to the number of arginines + number of lysines (NR/(NR + NK)) plotted against hydrophobicity (Eisenberg hydrophobicity scale) using the amino acid sequences of 39 cell-penetrating peptides and 1080 cationic antimicrobial peptides shows that a reduction in arginine content can be compensated for by an increase in both lysine and hydrophobic content.
; χ = -2 and Ao = 1.919 for Pn3m) is maximized near NR = 9, where observed membrane transduction activity is empirically highest. (F) ANTP, TAT peptide, R9 all induce the cubic Pn3m phase in membranes (DOPS∶DOPE = 20∶80 at P/L = 1/40). (G) The ratio of the number of arginines to the number of arginines + number of lysines (NR/(NR + NK)) plotted against hydrophobicity (Eisenberg hydrophobicity scale) using the amino acid sequences of 39 cell-penetrating peptides and 1080 cationic antimicrobial peptides shows that a reduction in arginine content can be compensated for by an increase in both lysine and hydrophobic content.
References
-
- Futaki S, et al. Arginine-rich peptides: An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. - PubMed
-
- Lewin M, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

