Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components
- PMID: 21969538
- PMCID: PMC3198349
- DOI: 10.1073/pnas.1110402108
Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components
Abstract
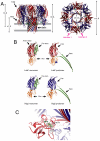
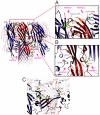
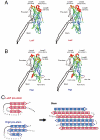
Staphylococcal γ-hemolysin is a bicomponent pore-forming toxin composed of LukF and Hlg2. These proteins are expressed as water-soluble monomers and then assemble into the oligomeric pore form on the target cell. Here, we report the crystal structure of the octameric pore form of γ-hemolysin at 2.5 Å resolution, which is the first high-resolution structure of a β-barrel transmembrane protein composed of two proteins reported to date. The octameric assembly consists of four molecules of LukF and Hlg2 located alternately in a circular pattern, which explains the biochemical data accumulated over the past two decades. The structure, in combination with the monomeric forms, demonstrates the elaborate molecular machinery involved in pore formation by two different molecules, in which interprotomer electrostatic interactions using loops connecting β2 and β3 (loop A: Asp43-Lys48 of LukF and Lys37-Lys43 of Hlg2) play pivotal roles as the structural determinants for assembly through unwinding of the N-terminal β-strands (amino-latch) of the adjacent protomer, releasing the transmembrane stem domain folded into a β-sheet in the monomer (prestem), and interaction with the adjacent protomer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Iacovache I, van der Goot FG, Pernot L. Pore formation: An ancient yet complex form of attack. Biochim Biophys Acta. 2008;1778:1611–1623. - PubMed
-
- Peitsch MC, Tschopp J. Assembly of macromolecular pores by immune defense systems. Curr Opin Cell Biol. 1991;3:710–716. - PubMed
-
- Gouaux E. Channel-forming toxins: tales of transformation. Curr Opin Struct Biol. 1997;7:566–573. - PubMed
-
- Parker MW, Feil SC. Pore-forming protein toxins: From structure to function. Prog Biophys Mol Biol. 2005;88:91–142. - PubMed
-
- Menestrina G, Serra MD, Prevost G. Mode of action of beta-barrel pore-forming toxins of the staphylococcal alpha-hemolysin family. Toxicon. 2001;39:1661–1672. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

