Molecular hijacking of siroheme for the synthesis of heme and d1 heme
- PMID: 21969545
- PMCID: PMC3215036
- DOI: 10.1073/pnas.1108228108
Molecular hijacking of siroheme for the synthesis of heme and d1 heme
Abstract
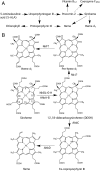
Modified tetrapyrroles such as chlorophyll, heme, siroheme, vitamin B(12), coenzyme F(430), and heme d(1) underpin a wide range of essential biological functions in all domains of life, and it is therefore surprising that the syntheses of many of these life pigments remain poorly understood. It is known that the construction of the central molecular framework of modified tetrapyrroles is mediated via a common, core pathway. Herein a further branch of the modified tetrapyrrole biosynthesis pathway is described in denitrifying and sulfate-reducing bacteria as well as the Archaea. This process entails the hijacking of siroheme, the prosthetic group of sulfite and nitrite reductase, and its processing into heme and d(1) heme. The initial step in these transformations involves the decarboxylation of siroheme to give didecarboxysiroheme. For d(1) heme synthesis this intermediate has to undergo the replacement of two propionate side chains with oxygen functionalities and the introduction of a double bond into a further peripheral side chain. For heme synthesis didecarboxysiroheme is converted into Fe-coproporphyrin by oxidative loss of two acetic acid side chains. Fe-coproporphyrin is then transformed into heme by the oxidative decarboxylation of two propionate side chains. The mechanisms of these reactions are discussed and the evolutionary significance of another role for siroheme is examined.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jensen RA. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. - PubMed
-
- Holliday GL, et al. Evolution of enzymes and pathways for the biosynthesis of cofactors. Nat Prod Rep. 2007;24:972–987. - PubMed
-
- Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. The biosynthesis of adenosylcobalamin vitamin B12. Nat Prod Rep. 2002;19:390–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBE0229441/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E024203/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 091163/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- BB/E022944/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E024203/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

