Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model
- PMID: 21969559
- PMCID: PMC3193202
- DOI: 10.1073/pnas.1108121108
Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model
Abstract
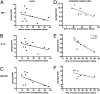
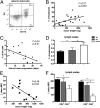
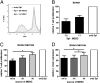
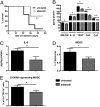
Tumor microenvironment is characterized by chronic inflammation represented by infiltrating leukocytes and soluble mediators, which lead to a local and systemic immunosuppression associated with cancer progression. Here, we used the ret transgenic spontaneous murine melanoma model that mimics human melanoma. Skin tumors and metastatic lymph nodes showed increased levels of inflammatory factors such as IL-1β, GM-CSF, and IFN-γ, which correlated with tumor progression. Moreover, Gr1(+)CD11b(+) myeloid-derived suppressor cells (MDSCs), known to inhibit tumor reactive T cells, were enriched in melanoma lesions and lymphatic organs during tumor progression. MDSC infiltration was associated with a strong TCR ζ-chain down-regulation in all T cells. Coculturing normal splenocytes with tumor-derived MDSC induced a decreased T-cell proliferation and ζ-chain expression, verifying the MDSC immunosuppressive function and suggesting that the tumor inflammatory microenvironment supports MDSC recruitment and immunosuppressive activity. Indeed, upon manipulation of the melanoma microenvironment with the phosphodiesterase-5 inhibitor sildenafil, we observed reduced levels of numerous inflammatory mediators (e.g., IL-1β, IL-6, VEGF, S100A9) in association with decreased MDSC amounts and immunosuppressive function, indicating an antiinflammatory effect of sildenafil. This led to a partial restoration of ζ-chain expression in T cells and to a significantly increased survival of tumor-bearing mice. CD8 T-cell depletion resulted in an abrogation of sildenafil beneficial outcome, suggesting the involvement of MDSC and CD8 T cells in the observed therapeutic effects. Our data imply that inhibition of chronic inflammation in the tumor microenvironment should be applied in conjunction with melanoma immunotherapies to increase their efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Spatz A, Batist G, Eggermont AM. The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol. 2010;22:163–168. - PubMed
-
- Guevara-Patiño JA, Turk MJ, Wolchok JD, Houghton AN. Immunity to cancer through immune recognition of altered self: Studies with melanoma. Adv Cancer Res. 2003;90:157–177. - PubMed
-
- Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: Past, present and future. Curr Opin Oncol. 2007;19:121–127. - PubMed
-
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. - PubMed
-
- Jandus C, Speiser D, Romero P. Recent advances and hurdles in melanoma immunotherapy. Pigment Cell Melanoma Res. 2009;22:711–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

