Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota
- PMID: 21969563
- PMCID: PMC3198331
- DOI: 10.1073/pnas.1107857108
Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota
Abstract
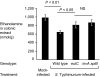
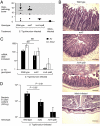
Conventional wisdom holds that microbes support their growth in vertebrate hosts by exploiting a large variety of nutrients. We show here that use of a specific nutrient (ethanolamine) confers a marked growth advantage on Salmonella enterica serovar Typhimurium (S. Typhimurium) in the lumen of the inflamed intestine. In the anaerobic environment of the gut, ethanolamine supports little or no growth by fermentation. However, S. Typhimurium is able to use this carbon source by inducing the gut to produce a respiratory electron acceptor (tetrathionate), which supports anaerobic growth on ethanolamine. The gut normally converts ambient hydrogen sulfide to thiosulfate, which it then oxidizes further to tetrathionate during inflammation. Evidence is provided that S. Typhimurium's growth advantage in an inflamed gut is because of its ability to respire ethanolamine, which is released from host tissue, but is not utilizable by competing bacteria. By inducing intestinal inflammation, S. Typhimurium sidesteps nutritional competition and gains the ability to use an abundant simple substrate, ethanolamine, which is provided by the host.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

