Modulation of canonical Wnt signaling by the extracellular matrix component biglycan
- PMID: 21969569
- PMCID: PMC3193219
- DOI: 10.1073/pnas.1110629108
Modulation of canonical Wnt signaling by the extracellular matrix component biglycan
Abstract
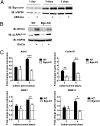
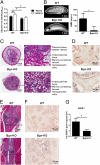
Although extracellular control of canonical Wnt signaling is crucial for tissue homeostasis, the role of the extracellular microenvironment in modulating this signaling pathway is largely unknown. In the present study, we show that a member of the small leucine-rich proteoglycan family, biglycan, enhances canonical Wnt signaling by mediating Wnt function via its core protein. Immunoprecipitation analysis revealed that biglycan interacts with both the canonical Wnt ligand Wnt3a and the Wnt coreceptor low-density lipoprotein receptor-related protein 6 (LRP6), possibly via the formation of a trimeric complex. Biglycan-deficient cells treated with exogenous Wnt3a had less Wnt3a retained in cell layers compared with WT cells. Furthermore, the Wnt-induced levels of LRP6 phosphorylation and expression of several Wnt target genes were blunted in biglycan-deficient cells. Both recombinant biglycan proteoglycan and biglycan core protein increased Wnt-induced β-catenin/T cell-specific factor-mediated transcriptional activity, and this activity was completely inhibited by Dickkopf 1. Interestingly, recombinant biglycan was able to rescue impaired Wnt signaling caused by a previously described missense mutation in the extracellular domain of human LRP6 (R611C). Furthermore, biglycan's modulation of canonical Wnt signaling affected the functional activities of osteoprogenitor cells, including the RUNX2-mediated transcriptional activity and calcium deposition. Use of a transplant system and a fracture healing model revealed that expression of Wnt-induced secreted protein 1 was decreased in bone formed by biglycan-deficient cells, further suggesting reduced Wnt signaling in vivo. We propose that biglycan may serve as a reservoir for Wnt in the pericellular space and modulate Wnt availability for activation of the canonical Wnt pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

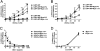
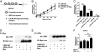
References
-
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. - PubMed
-
- Patel MS, Karsenty G. Regulation of bone formation and vision by LRP5. N Engl J Med. 2002;346:1572–1574. - PubMed
-
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

