Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism
- PMID: 21969575
- PMCID: PMC3193230
- DOI: 10.1073/pnas.1114042108
Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism
Abstract
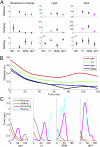
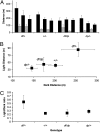
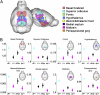
Recurrent copy number variations (CNVs) of human 16p11.2 have been associated with a variety of developmental/neurocognitive syndromes. In particular, deletion of 16p11.2 is found in patients with autism, developmental delay, and obesity. Patients with deletions or duplications have a wide range of clinical features, and siblings carrying the same deletion often have diverse symptoms. To study the consequence of 16p11.2 CNVs in a systematic manner, we used chromosome engineering to generate mice harboring deletion of the chromosomal region corresponding to 16p11.2, as well as mice harboring the reciprocal duplication. These 16p11.2 CNV models have dosage-dependent changes in gene expression, viability, brain architecture, and behavior. For each phenotype, the consequence of the deletion is more severe than that of the duplication. Of particular note is that half of the 16p11.2 deletion mice die postnatally; those that survive to adulthood are healthy and fertile, but have alterations in the hypothalamus and exhibit a "behavior trap" phenotype-a specific behavior characteristic of rodents with lateral hypothalamic and nigrostriatal lesions. These findings indicate that 16p11.2 CNVs cause brain and behavioral anomalies, providing insight into human neurodevelopmental disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Moeschler JB. Medical genetics diagnostic evaluation of the child with global developmental delay or intellectual disability. Curr Opin Neurol. 2008;21:117–122. - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

