Transcriptional modulator H2A histone family, member Y (H2AFY) marks Huntington disease activity in man and mouse
- PMID: 21969577
- PMCID: PMC3193232
- DOI: 10.1073/pnas.1104409108
Transcriptional modulator H2A histone family, member Y (H2AFY) marks Huntington disease activity in man and mouse
Abstract
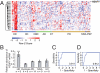
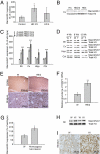
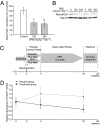
Huntington disease (HD) is a progressive neurodegenerative disease that affects 30,000 individuals in North America. Treatments that slow its relentless course are not yet available, and biomarkers that can reliably measure disease activity and therapeutic response are urgently needed to facilitate their development. Here, we interrogated 119 human blood samples for transcripts associated with HD. We found that the dynamic regulator of chromatin plasticity H2A histone family, member Y (H2AFY) is specifically overexpressed in the blood and frontal cortex of patients with HD compared with controls. This association precedes the onset of clinical symptoms, was confirmed in two mouse models, and was independently replicated in cross-sectional and longitudinal clinical studies comprising 142 participants. A histone deacetylase inhibitor that suppresses neurodegeneration in animal models reduces H2AFY levels in a randomized phase II clinical trial. This study identifies the chromatin regulator H2AFY as a potential biomarker associated with disease activity and pharmacodynamic response that may become useful for enabling disease-modifying therapeutics for HD.
Conflict of interest statement
Conflict of interest statement: C.R.S. has served as a consultant to Link Medicine Corporation and was a scientific collaborator of DiaGenic in a study funded by the Michael J. Fox Foundation. He is listed as coinventor on US patent/applications held by the Brigham and Women's Hospital relating to diagnostics in neurodegenerative diseases. S.M.H. is a scientific collaborator with the Novartis Institutes for Biomedical Research. He is listed as coinventor on US patent/applications held by the Massachusetts General Hospital relating to therapeutics and diagnostics in neurodegenerative diseases.
Figures
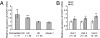
Comment in
-
Chromatin plasticity and the pathogenesis of Huntington disease.Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):16867-8. doi: 10.1073/pnas.1113321108. Epub 2011 Oct 3. Proc Natl Acad Sci U S A. 2011. PMID: 21969556 Free PMC article. No abstract available.
-
Does chromatin modulation provide the first wet biomarker for Huntington's disease?Mov Disord. 2012 Apr;27(4):473. doi: 10.1002/mds.24914. Mov Disord. 2012. PMID: 22590715 No abstract available.
References
-
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003;19:233–238. - PubMed
-
- van der Burg JMM, Björkqvist M, Brundin P. Beyond the brain: Widespread pathology in Huntington's disease. Lancet Neurol. 2009;8:765–774. - PubMed
-
- Panov AV, et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. - PubMed
-
- Maglione V, et al. The platelet maximum number of A2A-receptor binding sites (Bmax) linearly correlates with age at onset and CAG repeat expansion in Huntington's disease patients with predominant chorea. Neurosci Lett. 2006;393:27–30. - PubMed
-
- Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J Neurochem. 2005;93:611–623. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

