CCAAT/enhancer binding protein alpha (C/EBP(alpha))-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation
- PMID: 21969581
- PMCID: PMC3193237
- DOI: 10.1073/pnas.1112169108
CCAAT/enhancer binding protein alpha (C/EBP(alpha))-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation
Erratum in
- Proc Natl Acad Sci U S A. 2012 Jul 3;109(27):11053
- Proc Natl Acad Sci U S A. 2012. doi: 10.1073/pnas.1208762109. Castellano, Giancarlo [added] doi: 10.1073/pnas.1208762109
Abstract
Earlier work has shown that pre-B cells can be converted into macrophages by the transcription factor CCAAT/enhancer binding protein α at very high frequencies. Using this system, we performed a systematic analysis of whether during transdifferentiation the cells transiently reactivate progenitor-restricted genes or even retrodifferentiate. A transcriptome analysis of transdifferentiating cells showed that most genes are up- or down-regulated continuously, acquiring a macrophage phenotype within 5 d. In addition, we observed the transient reactivation of a subset of immature myeloid markers, as well as low levels of the progenitor markers Kit and FMS-like tyrosine kinase 3 and a few lineage-inappropriate genes. Importantly, however, we were unable to observe the reexpression of cell-surface marker combinations that characterize hematopoietic stem and progenitor cells, including c-Kit and FMS-like tyrosine kinase 3, even when CAAT/enhancer binding protein α was activated in pre-B cells under culture conditions that favor growth of hematopoietic stem and progenitor cells or when the transcription factor was activated in a time-limited fashion. Together, our findings are consistent with the notion that the conversion from pre-B cells to macrophages is mostly direct and does not involve overt retrodifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


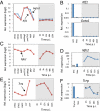
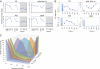
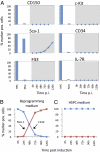
References
-
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. - PubMed
-
- Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. - PubMed
-
- Huang P, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. - PubMed
-
- Zhou Q, Melton DA. Extreme makeover: Converting one cell into another. Cell Stem Cell. 2008;3:382–388. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

