Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74
- PMID: 21969590
- PMCID: PMC3198363
- DOI: 10.1073/pnas.1107023108
Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74
Abstract
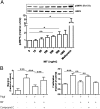
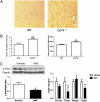
Macrophage migration inhibitory factor (MIF) is a pleiotropic inflammatory cytokine that has been implicated in various inflammatory diseases. Chronic inflammation is a mainstay of liver fibrosis, a leading cause of morbidity worldwide, but the role of MIF in liver scarring has not yet been elucidated. Here we have uncovered an unexpected antifibrotic role for MIF. Mice genetically deleted in Mif (Mif(-/-)) showed strongly increased fibrosis in two models of chronic liver injury. Pronounced liver fibrosis in Mif(-/-) mice was associated with alterations in fibrosis-relevant genes, but not by a changed intrahepatic immune cell infiltration. Next, a direct impact of MIF on hepatic stellate cells (HSC) was assessed in vitro. Although MIF alone had only marginal effects on HSCs, it markedly inhibited PDGF-induced migration and proliferation of these cells. The inhibitory effects of MIF were mediated by CD74, which we detected as the most abundant known MIF receptor on HSCs. MIF promoted the phosphorylation of AMP-activated protein kinase (AMPK) in a CD74-dependent manner and, in turn, inhibition of AMPK reversed the inhibition of PDGF-induced HSC activation by MIF. The pivotal role of CD74 in MIF-mediated antifibrotic properties was further supported by augmented liver scarring of Cd74(-/-) mice. Moreover, mice treated with recombinant MIF displayed a reduced fibrogenic response in vivo. In conclusion, we describe a previously unexplored antifibrotic function of MIF that is mediated by the CD74/AMPK signaling pathway in HSCs. The results imply MIF and CD74 as targets for treatment of liver diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Antifibrotic role of macrophage migration inhibitory factor: discovery of an unexpected function.Hepatology. 2012 Apr;55(4):1295-7. doi: 10.1002/hep.25605. Hepatology. 2012. PMID: 22461077 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

