Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells
- PMID: 21969607
- PMCID: PMC3233034
- DOI: 10.1128/MCB.06120-11
Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells
Abstract
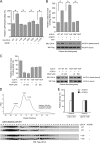
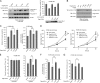
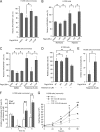
The Warburg effect describes an increase in aerobic glycolysis and enhanced lactate production in cancer cells. Lactate dehydrogenase A (LDH-A) regulates the last step of glycolysis that generates lactate and permits the regeneration of NAD(+). LDH-A gene expression is believed to be upregulated by both HIF and Myc in cancer cells to achieve increased lactate production. However, how oncogenic signals activate LDH-A to regulate cancer cell metabolism remains unclear. We found that the oncogenic receptor tyrosine kinase FGFR1 directly phosphorylates LDH-A. Phosphorylation at Y10 and Y83 enhances LDH-A activity by enhancing the formation of active, tetrameric LDH-A and the binding of LDH-A substrate NADH, respectively. Moreover, Y10 phosphorylation of LDH-A is common in diverse human cancer cells, which correlates with activation of multiple oncogenic tyrosine kinases. Interestingly, cancer cells with stable knockdown of endogenous LDH-A and rescue expression of a catalytic hypomorph LDH-A mutant, Y10F, demonstrate increased respiration through mitochondrial complex I to sustain glycolysis by providing NAD(+). However, such a compensatory increase in mitochondrial respiration in Y10F cells is insufficient to fully sustain glycolysis. Y10 rescue cells show decreased cell proliferation and ATP levels under hypoxia and reduced tumor growth in xenograft nude mice. Our findings suggest that tyrosine phosphorylation enhances LDH-A enzyme activity to promote the Warburg effect and tumor growth by regulating the NADH/NAD(+) redox homeostasis, representing an acute molecular mechanism underlying the enhanced lactate production in cancer cells.
Figures




References
-
- Brahimi-Horn M. C., Chiche J., Pouyssegur J. 2007. Hypoxia signalling controls metabolic demand. Curr. Opin. Cell Biol. 19:223–229 - PubMed
-
- Bui T., Thompson C. B. 2006. Cancer's sweet tooth. Cancer Cell 9:419–420 - PubMed
-
- Dang C. V. 2009. PKM2 tyrosine phosphorylation and glutamine metabolism signal a different view of the Warburg effect. Sci. Signal. 2:pe75. - PubMed
-
- Durisova V., Vrbanova A., Ziegelhoffer A., Breier A. 1990. Interaction of Cibacron Blue 3GA and Remazol Brilliant Blue R with the nucleotide binding site of lactate dehydrogenase and (Na+ + K+)-ATPase. Gen. Physiol. Biophys. 9:519–528 - PubMed
-
- Fantin V. R., St-Pierre J., Leder P. 2006. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9:425–434 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
