Evolutionary diversification of Mesobuthus α-scorpion toxins affecting sodium channels
- PMID: 21969612
- PMCID: PMC3270107
- DOI: 10.1074/mcp.M111.012054
Evolutionary diversification of Mesobuthus α-scorpion toxins affecting sodium channels
Abstract
α-Scorpion toxins constitute a family of peptide modulators that induce a prolongation of the action potential of excitable cells by inhibiting voltage-gated sodium channel inactivation. Although they all adopt a conserved structural scaffold, the potency and phylogentic preference of these toxins largely vary, which render them an intriguing model for studying evolutionary diversification among family members. Here, we report molecular characterization of a new multigene family of α-toxins comprising 13 members (named MeuNaTxα-1 to MeuNaTxα-13) from the scorpion Mesobuthus eupeus. Of them, five native toxins (MeuNaTxα-1 to -5) were purified to homogeneity from the venom and the solution structure of MeuNaTxα-5 was solved by nuclear magnetic resonance. A systematic functional evaluation of MeuNaTxα-1, -2, -4, and -5 was conducted by two-electrode voltage-clamp recordings on seven cloned mammalian voltage-gated sodium channels (Na(v)1.2 to Na(v)1.8) and the insect counterpart DmNa(v)1 expressed in Xenopus oocytes. Results show that all these four peptides slow inactivation of DmNa(v)1 and are inactive on Na(v)1.8 at micromolar concentrations. However, they exhibit differential specificity for the other six channel isoforms (Na(v)1.2 to Na(v)1.7), in which MeuNaTxα-4 shows no activity on these isoforms and thus represents the first Mesobuthus-derived insect-selective α-toxin identified so far with a half maximal effective concentration of 130 ± 2 nm on DmNa(v)1 and a half maximal lethal dose of about 200 pmol g(-1) on the insect Musca domestica; MeuNaTxα-2 only affects Na(v)1.4; MeuNaTxα-1 and MeuNaTxα-5 have a wider range of channel spectrum, the former active on Na(v)1.2, Na(v)1.3, Na(v)1.6, and Na(v)1.7, whereas the latter acting on Na(v)1.3-Na(v)1.7. Remarkably, MeuNaTxα-4 and MeuNaTxα-5 are two nearly identical peptides differing by only one point mutation at site 50 (A50V) but exhibit rather different channel subtype selectivity, highlighting a switch role of this site in altering the target specificity. By the maximum likelihood models of codon substitution, we detected nine positively selected sites (PSSs) that could be involved in functional diversification of Mesobuthus α-toxins. The PSSs include site 50 and other seven sites located in functional surfaces of α-toxins. This work represents the first thorough investigation of evolutionary diversification of α-toxins derived from a specific scorpion lineage from the perspectives of sequence, structure, function, and evolution.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures

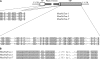

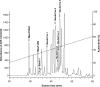
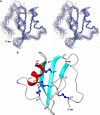



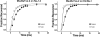


References
-
- Rochat H., Martin-Eauclaire M. F. (2000) Animal Toxins: Facts and Protocols. Birkhauser Verlag, Basel, Boston, Berlin
-
- Billen B., Bosmans F., Tytgat J. (2008) Animal peptides targeting voltage-activated sodium channels. Curr. Pharm. Design. 14, 2492–2502 - PubMed
-
- Lewis R. J., Garcia M. L. (2003) Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2, 790–802 - PubMed
-
- Catterall W. A. (1995) Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 64, 493–531 - PubMed
-
- Catterall W. A. (2000) From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 26, 13–25 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

