Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain
- PMID: 21969850
- PMCID: PMC3182161
- DOI: 10.1371/journal.pone.0023059
Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain
Abstract
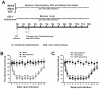
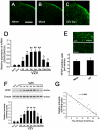
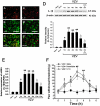
Postherpetic neuralgia (PHN), the most common complication of herpes zoster (HZ), plays a major role in decreased life quality of HZ patients. However, the neural mechanisms underlying PHN remain unclear. Here, using a PHN rat model at 2 weeks after varicella zoster virus infection, we found that spinal astrocytes were dramatically activated. The mechanical allodynia and spinal central sensitization were significantly attenuated by intrathecally injected L-α-aminoadipate (astrocytic specific inhibitor) whereas minocycline (microglial specific inhibitor) had no effect, which indicated that spinal astrocyte but not microglia contributed to the chronic pain in PHN rat. Further study was taken to investigate the molecular mechanism of astrocyte-incudced allodynia in PHN rat at post-infection 2 weeks. Results showed that nitric oxide (NO) produced by inducible nitric oxide synthase mediated the development of spinal astrocytic activation, and activated astrocytes dramatically increased interleukin-1β expression which induced N-methyl-D-aspartic acid receptor (NMDAR) phosphorylation in spinal dorsal horn neurons to strengthen pain transmission. Taken together, these results suggest that spinal activated astrocytes may be one of the most important factors in the pathophysiology of PHN and "NO-Astrocyte-Cytokine-NMDAR-Neuron" pathway may be the detailed neural mechanisms underlying PHN. Thus, inhibiting spinal astrocytic activation may represent a novel therapeutic strategy for clinical management of PHN.
Conflict of interest statement
Figures




Similar articles
-
Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model.PLoS One. 2013 Apr 9;8(4):e60733. doi: 10.1371/journal.pone.0060733. Print 2013. PLoS One. 2013. PMID: 23585846 Free PMC article.
-
Spinal astrocytic activation contributes to mechanical allodynia in a mouse model of type 2 diabetes.Brain Res. 2011 Jan 12;1368:324-35. doi: 10.1016/j.brainres.2010.10.044. Epub 2010 Nov 12. Brain Res. 2011. PMID: 20971097
-
Involvement of trigeminal astrocyte activation in masseter hyperalgesia under stress.Physiol Behav. 2015 Apr 1;142:57-65. doi: 10.1016/j.physbeh.2015.02.005. Epub 2015 Feb 3. Physiol Behav. 2015. PMID: 25660342
-
Management of herpes zoster (shingles) and postherpetic neuralgia.Expert Opin Pharmacother. 2004 Mar;5(3):551-9. doi: 10.1517/14656566.5.3.551. Expert Opin Pharmacother. 2004. PMID: 15013924 Review.
-
Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient.Drugs Aging. 2008;25(12):991-1006. doi: 10.2165/0002512-200825120-00002. Drugs Aging. 2008. PMID: 19021299 Review.
Cited by
-
Contribution of spinal cord glial cells to L. amazonensis experimental infection-induced pain in BALB/c mice.J Neuroinflammation. 2019 May 28;16(1):113. doi: 10.1186/s12974-019-1496-2. J Neuroinflammation. 2019. PMID: 31138231 Free PMC article.
-
Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia.Nutrients. 2020 Aug 9;12(8):2384. doi: 10.3390/nu12082384. Nutrients. 2020. PMID: 32784896 Free PMC article.
-
Comparing Gene Expression in the Parabrachial and Amygdala of Diestrus and Proestrus Female Rats after Orofacial Varicella Zoster Injection.Int J Mol Sci. 2020 Aug 11;21(16):5749. doi: 10.3390/ijms21165749. Int J Mol Sci. 2020. PMID: 32796585 Free PMC article.
-
The analgesic effect of early hyperbaric oxygen treatment in chronic constriction injury rats and its influence on nNOS and iNOS expression and inflammatory factor production.Mol Pain. 2018 Jan-Dec;14:1744806918765837. doi: 10.1177/1744806918765837. Mol Pain. 2018. PMID: 29592784 Free PMC article.
-
σ1 receptors activate astrocytes via p38 MAPK phosphorylation leading to the development of mechanical allodynia in a mouse model of neuropathic pain.Br J Pharmacol. 2014 Dec;171(24):5881-97. doi: 10.1111/bph.12893. Epub 2014 Nov 24. Br J Pharmacol. 2014. PMID: 25158784 Free PMC article.
References
-
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–251. - PubMed
-
- Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: a self-limited disease with severe impact. Neurology. 1995;45:S52–53. - PubMed
-
- Bowsher D. Pathophysiology of postherpetic neuralgia: towards a rational treatment. Neurology. 1995;45(Suppl. 8):S56–S57. - PubMed
-
- Gilden DH. Herpes zoster with postherpetic neuralgia: persisting pain and frustration. N Engl J Med. 1994;330:932–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

