Engraftment of insulin-producing cells from porcine islets in non-immune-suppressed rats or nonhuman primates transplanted previously with embryonic pig pancreas
- PMID: 21969909
- PMCID: PMC3182564
- DOI: 10.1155/2011/261352
Engraftment of insulin-producing cells from porcine islets in non-immune-suppressed rats or nonhuman primates transplanted previously with embryonic pig pancreas
Abstract
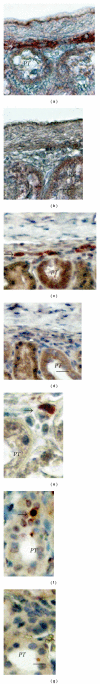
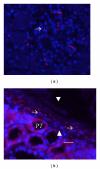
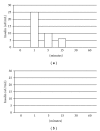
Transplantation therapy for diabetes is limited by unavailability of donor organs and outcomes complicated by immunosuppressive drug toxicity. Xenotransplantation is a strategy to overcome supply problems. Implantation of tissue obtained early during embryogenesis is a way to reduce transplant immunogenicity. Insulin-producing cells originating from embryonic pig pancreas obtained very early following pancreatic primordium formation (embryonic day 28 (E28)) engraft long-term in non-immune, suppressed diabetic rats or rhesus macaques. Morphologically, similar cells originating from adult porcine islets of Langerhans (islets) engraft in non-immune-suppressed rats or rhesus macaques previously transplanted with E28 pig pancreatic primordia. Our data are consistent with induction of tolerance to an endocrine cell component of porcine islets induced by previous transplantation of embryonic pig pancreas, a novel finding we designate organogenetic tolerance. The potential exists for its use to enable the use of pigs as islet cell donors for humans with no immune suppression requirement.
Figures


Similar articles
-
Development of a novel xenotransplantation strategy for treatment of diabetes mellitus in rat hosts and translation to non-human primates.Organogenesis. 2012 Apr-Jun;8(2):41-8. doi: 10.4161/org.20930. Epub 2012 Apr 1. Organogenesis. 2012. PMID: 22699748 Free PMC article. Review.
-
Organogenetic tolerance.Organogenesis. 2010 Oct-Dec;6(4):270-5. doi: 10.4161/org.6.4.13283. Organogenesis. 2010. PMID: 21220963 Free PMC article. Review.
-
Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques.Organogenesis. 2011 Jul-Sep;7(3):154-62. doi: 10.4161/org.7.3.16522. Epub 2011 Jul 1. Organogenesis. 2011. PMID: 21654197 Free PMC article.
-
Xenotransplantation of embryonic pig pancreas for treatment of diabetes mellitus in non-human primates.J Biomed Sci Eng. 2013 May 1;6(5A):10.4236/jbise.2013.65A002. doi: 10.4236/jbise.2013.65A002. J Biomed Sci Eng. 2013. PMID: 24312695 Free PMC article.
-
Engraftment of cells from porcine islets of Langerhans and normalization of glucose tolerance following transplantation of pig pancreatic primordia in nonimmune-suppressed diabetic rats.Am J Pathol. 2010 Aug;177(2):854-64. doi: 10.2353/ajpath.2010.091193. Epub 2010 Jun 25. Am J Pathol. 2010. PMID: 20581052 Free PMC article.
Cited by
-
Development of a novel xenotransplantation strategy for treatment of diabetes mellitus in rat hosts and translation to non-human primates.Organogenesis. 2012 Apr-Jun;8(2):41-8. doi: 10.4161/org.20930. Epub 2012 Apr 1. Organogenesis. 2012. PMID: 22699748 Free PMC article. Review.
References
-
- Brands K, Colvin E, Williams LJ, Wang R, Lock RB, Tuch BE. Reduced immunogenicity of first-trimester human fetal pancreas. Diabetes. 2008;57(3):627–634. - PubMed
-
- Rogers SA, Chen F, Talcott M, Hammerman MR. Islet cell engraftment and control of diabetes in rats after transplantation of pig pancreatic anlagen. American Journal of Physiology. 2004;286(4):E502–E509. - PubMed
-
- Rogers SA, Liapis H, Hammerman MR. Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transplant Immunology. 2005;14(2):67–75. - PubMed
-
- Rogers SA, Chen F, Talcott M, Liapis H, Hammerman MR. Glucose tolerance normalization following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic ZDF rats. Transplant Immunology. 2006;16(3-4):176–184. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

