Use of a laboratory information system driven tool for pre-signout quality assurance of random cytopathology reports
- PMID: 21969923
- PMCID: PMC3169920
- DOI: 10.4103/2153-3539.84279
Use of a laboratory information system driven tool for pre-signout quality assurance of random cytopathology reports
Abstract
Background: Quality assurance (QA) programs in cytopathology laboratories in the USA currently primarily involve the review of Pap tests per clinical laboratory improvement amendments of 1988 federal regulations. A pre-signout quality assurance tool (PQAT) at our institution allows the laboratory information system (LIS) to also automatically and randomly select an adjustable percentage of non-gynecological cytopathology cases for review before release of the final report. The aim of this study was to review our experience and the effectiveness of this novel PQAT tool in cytology.
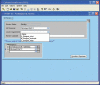
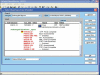
Materials and methods: Software modifications in the existing LIS application (CoPathPlus, Cerner) allow for the random QA of 8% of cases prior to signout. Selected cases are assigned to a second QA cytopathologist for review and all agreement and disagreements tracked. Detected errors are rectified before the case is signed out. Data from cases selected for PQAT over an 18-month period were collected and analyzed.
Results: The total number of non-gynecological cases selected for QA review was 1339 (7.45%) out of 17,967 cases signed out during this time period. Most (1304) cases (97.4%) had an agreement in diagnosis. In 2.6% of cases, there were disagreements, including 34 minor and only 1 major disagreement. Average turnaround time of cases selected for review was not significantly altered.
Conclusion: The PQAT provides a prospective QA mechanism in non-gynecological cytopathology to prevent diagnostic errors from occurring. This LIS-driven tool allows for peer review and corrective action to be taken prior to reporting without delaying turnaround time, thereby improving patient safety.
Keywords: Cytopathology; error; laboratory information system; patient safety; quality assurance.
Figures
References
-
- Association of Directors of Anatomic and Surgical Pathology. Recommendations for quality assurance and improvement in surgical and autopsy pathology. Am J Surg Pathol. 2006;30:1469–71. - PubMed
-
- Frable WJ. Surgical pathology--second reviews, institutional reviews, audits, and correlations: what's out there? Error or diagnostic variation? Arch Pathol Lab Med. 2006;130:620–5. - PubMed
-
- Manion E, Cohen MB, Weydert J. Mandatory second opinion in surgical pathology referral material: clinical consequences of major disagreements. Am J Surg Pathol. 2008;32:732–7. - PubMed
-
- Cibas ES. Laboratory management. In: Cibas ES, Ducatman BS, editors. Cytology. Diagnostic principles and clinical correlates. 3rd ed. Vol. 17. Philadelphia, PA: Saunders Elsevier; 2009. pp. 495–521.
-
- Tabbara SO, Sidawy MK. Evaluation of the 10% rescreen of negative gynecologic smears as a quality assurance measure. Diagn Cytopathol. 1996;14:84–6. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

