Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycin
- PMID: 21970554
- PMCID: PMC3198935
- DOI: 10.1186/1465-9921-12-131
Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycin
Abstract
Background: Cluster of differentiation 69 (CD69), an early activation marker antigen on T and B cells, is also expressed on activated macrophages and neutrophils, suggesting that CD69 may play a role in inflammatory diseases. To determine the effect of CD69 deficiency on bleomycin(BLM)-induced lung injury, we evaluated the inflammatory response following intratracheal BLM administration and the subsequent fibrotic changes in wild type (WT) and CD69-deficient (CD69-/-) mice.
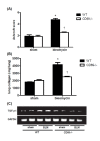
Methods: The mice received a single dose of 3 mg/kg body weight of BLM and were sacrificed at 7 or 14 days post-instillation (dpi). Lung inflammation in the acute phase (7 dpi) was investigated by differential cell counts and cytokine array analyses of bronchoalveolar lavage fluid. In addition, lung fibrotic changes were evaluated at 14 dpi by histopathology and collagen assays. We also used reverse transcription polymerase chain reaction to measure the mRNA expression level of transforming growth factor β1 (TGF-β1) in the lungs of BLM-treated mice.
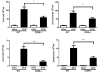
Results: CD69-/- mice exhibited less lung damage than WT mice, as shown by reductions in the following indices: (1) loss of body weight, (2) wet/dry ratio of lung, (3) cytokine levels in BALF, (4) histological evidence of lung injury, (5) lung collagen deposition, and (6) TGF-β1 mRNA expression in the lung.
Conclusion: The present study clearly demonstrates that CD69 plays an important role in the progression of lung injury induced by BLM.
Figures
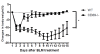




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

