Improved retroviral suicide gene transfer in colon cancer cell lines after cell synchronization with methotrexate
- PMID: 21970612
- PMCID: PMC3199255
- DOI: 10.1186/1756-9966-30-92
Improved retroviral suicide gene transfer in colon cancer cell lines after cell synchronization with methotrexate
Abstract
Background: Cancer gene therapy by retroviral vectors is mainly limited by the level of transduction. Retroviral gene transfer requires target cell division. Cell synchronization, obtained by drugs inducing a reversible inhibition of DNA synthesis, could therefore be proposed to precondition target cells to retroviral gene transfer. We tested whether drug-mediated cell synchronization could enhance the transfer efficiency of a retroviral-mediated gene encoding herpes simplex virus thymidine kinase (HSV-tk) in two colon cancer cell lines, DHDK12 and HT29.
Methods: Synchronization was induced by methotrexate (MTX), aracytin (ara-C) or aphidicolin. Gene transfer efficiency was assessed by the level of HSV-TK expression. Transduced cells were driven by ganciclovir (GCV) towards apoptosis that was assessed using annexin V labeling by quantitative flow cytometry.
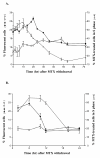
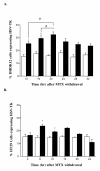
Results: DHDK12 and HT29 cells were synchronized in S phase with MTX but not ara-C or aphidicolin. In synchronized DHDK12 and HT29 cells, the HSV-TK transduction rates were 2 and 1.5-fold higher than those obtained in control cells, respectively. Furthermore, the rate of apoptosis was increased two-fold in MTX-treated DHDK12 cells after treatment with GCV.
Conclusions: Our findings indicate that MTX-mediated synchronization of target cells allowed a significant improvement of retroviral HSV-tk gene transfer, resulting in an increased cell apoptosis in response to GCV. Pharmacological control of cell cycle may thus be a useful strategy to optimize the efficiency of retroviral-mediated cancer gene therapy.
Figures



Similar articles
-
In situ generation of pseudotyped retroviral progeny by adenovirus-mediated transduction of tumor cells enhances the killing effect of HSV-tk suicide gene therapy in vitro and in vivo.J Gene Med. 2004 Mar;6(3):288-99. doi: 10.1002/jgm.490. J Gene Med. 2004. PMID: 15026990
-
Retrovirus-mediated transfer of a suicide gene into lens epithelial cells in vitro and in an experimental model of posterior capsule opacification.Curr Eye Res. 1999 Dec;19(6):472-82. doi: 10.1076/ceyr.19.6.472.5284. Curr Eye Res. 1999. PMID: 10550788
-
Mechanisms for ganciclovir resistance in gastrointestinal tumor cells transduced with a retroviral vector containing the herpes simplex virus thymidine kinase gene.Clin Cancer Res. 1998 Mar;4(3):731-41. Clin Cancer Res. 1998. PMID: 9533543
-
Molecular mechanism for ganciclovir resistance in human T lymphocytes transduced with retroviral vectors carrying the herpes simplex virus thymidine kinase gene.Blood. 2001 Jan 1;97(1):122-9. doi: 10.1182/blood.v97.1.122. Blood. 2001. PMID: 11133751
-
Retroviral transfer of HSV1-TK gene into human lung cancer cell line.J Mol Med (Berl). 1995 Mar;73(3):107-12. doi: 10.1007/BF00198237. J Mol Med (Berl). 1995. PMID: 7633946 Review.
Cited by
-
Safety and efficacy of suicide gene therapy with adenosine deaminase 5-fluorocytosine silmutaneously in in vitro cultures of melanoma and retinal cell lines.J Cancer. 2014 Apr 17;5(5):368-81. doi: 10.7150/jca.9147. eCollection 2014. J Cancer. 2014. PMID: 24799955 Free PMC article.
-
A Brief Introduction to Current Cancer Gene Therapy.Methods Mol Biol. 2022;2521:1-21. doi: 10.1007/978-1-0716-2441-8_1. Methods Mol Biol. 2022. PMID: 35732990
-
Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging.Cell Cycle. 2016 Jul 2;15(13):1715-23. doi: 10.1080/15384101.2016.1181240. Epub 2016 May 6. Cell Cycle. 2016. PMID: 27152859 Free PMC article.
-
pH dependent poly[2-(methacryloyloxyethyl)trimetylammonium chloride-co-methacrylic acid]hydrogels for enhanced targeted delivery of 5-fluorouracil in colon cancer cells.J Mater Sci Mater Med. 2014 Apr;25(4):999-1012. doi: 10.1007/s10856-013-5132-x. Epub 2014 Jan 8. J Mater Sci Mater Med. 2014. PMID: 24398912
-
Suicide Gene Therapy for Cancer - Current Strategies.J Genet Syndr Gene Ther. 2013 Aug 9;4:16849. doi: 10.4172/2157-7412.1000139. J Genet Syndr Gene Ther. 2013. PMID: 24294541 Free PMC article.
References
-
- Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, Hurskainen H, Tyynela K, Turunen M, Vanninen R, Lehtolainen P, Paljarvi L, Johansson R, Vapalahti M, Yla-Herttuala S. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. - DOI - PubMed
-
- Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

