Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish
- PMID: 21971049
- PMCID: PMC3243515
- DOI: 10.1074/jbc.M111.301093
Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish
Abstract
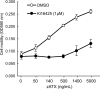
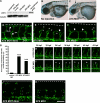
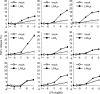
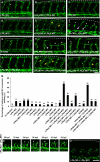
Autotaxin (ATX) is a multifunctional ecto-type phosphodiesterase that converts lysophospholipids, such as lysophosphatidylcholine, to lysophosphatidic acid (LPA) by its lysophospholipase D activity. LPA is a lipid mediator with diverse biological functions, most of which are mediated by G protein-coupled receptors specific to LPA (LPA1-6). Recent studies on ATX knock-out mice revealed that ATX has an essential role in embryonic blood vessel formation. However, the underlying molecular mechanisms remain to be solved. A data base search revealed that ATX and LPA receptors are conserved in wide range of vertebrates from fishes to mammals. Here we analyzed zebrafish ATX (zATX) and LPA receptors both biochemically and functionally. zATX, like mammalian ATX, showed lysophospholipase D activity to produce LPA. In addition, all zebrafish LPA receptors except for LPA5a and LPA5b were found to respond to LPA. Knockdown of zATX in zebrafish embryos by injecting morpholino antisense oligonucleotides (MOs) specific to zATX caused abnormal blood vessel formation, which has not been observed in other morphant embryos or mutants with vascular defects reported previously. In ATX morphant embryos, the segmental arteries sprouted normally from the dorsal aorta but stalled in midcourse, resulting in aberrant vascular connection around the horizontal myoseptum. Similar vascular defects were not observed in embryos in which each single LPA receptor was attenuated by using MOs. Interestingly, similar vascular defects were observed when both LPA1 and LPA4 functions were attenuated by using MOs and/or a selective LPA receptor antagonist, Ki16425. These results demonstrate that the ATX-LPA-LPAR axis is a critical regulator of embryonic vascular development that is conserved in vertebrates.
Figures



References
-
- Stracke M. L., Krutzsch H. C., Unsworth E. J., Arestad A., Cioce V., Schiffmann E., Liotta L. A. (1992) J. Biol. Chem. 267, 2524–2529 - PubMed
-
- Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., Fukuzawa K. (2002) J. Biol. Chem. 277, 39436–39442 - PubMed
-
- Aoki J. (2004) Semin. Cell Dev. Biol. 15, 477–489 - PubMed
-
- Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., Arai H. (2006) J. Biol. Chem. 281, 25822–25830 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

