Opiate versus psychostimulant addiction: the differences do matter
- PMID: 21971065
- PMCID: PMC3721140
- DOI: 10.1038/nrn3104
Opiate versus psychostimulant addiction: the differences do matter
Abstract
The publication of the psychomotor stimulant theory of addiction in 1987 and the finding that addictive drugs increase dopamine concentrations in the rat mesolimbic system in 1988 have led to a predominance of psychobiological theories that consider addiction to opiates and addiction to psychostimulants as essentially identical phenomena. Indeed, current theories of addiction - hedonic allostasis, incentive sensitization, aberrant learning and frontostriatal dysfunction - all argue for a unitary account of drug addiction. This view is challenged by behavioural, cognitive and neurobiological findings in laboratory animals and humans. Here, we argue that opiate addiction and psychostimulant addiction are behaviourally and neurobiologically distinct and that the differences have important implications for addiction treatment, addiction theories and future research.
Figures
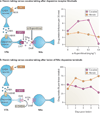
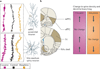


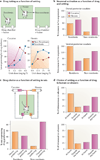
References
-
- Collier HO. Supersensitivity and dependence. Nature. 1968;220:228–231. - PubMed
-
- Collier HO. Supersensitivity and dependence on cocaine. Nature. 1968;220:1327–1328. - PubMed
-
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J. Abnorm. Psychol. 1973;81:158–171. - PubMed
-
- Olds J, Milner PM. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954;47:419–427. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

