Early antiretroviral therapy in HIV-1-infected infants, 1996-2008: treatment response and duration of first-line regimens
- PMID: 21971357
- PMCID: PMC3433031
- DOI: 10.1097/QAD.0b013e32834d614c
Early antiretroviral therapy in HIV-1-infected infants, 1996-2008: treatment response and duration of first-line regimens
Abstract
Objective: To investigate virological and immunological response to antiretroviral therapy (ART), and predictors of switching and interrupting treatment among infants starting ART across Europe.
Design: Cohort study.
Methods: Nine cohorts from 13 European countries contributed data on HIV-infected infants born 1996-2008 and starting ART before age 12 months. Logistic and linear regression, and competing risks methods were used to assess predictors of virological (viral load <400 copies/ml) and immunological (change in CD4 Z-score) response, switching to second-line ART and treatment interruptions with viral load less than 400 copies/ml.
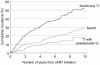
Results: A total of 437 infants were followed for median 5.9 (interquartile range 2.3-7.6) years after starting ART; 30% had an AIDS diagnosis prior to ART initiation. 53% had suppressed viral load <400 copies/ml at 12 months in 1996-1999, increasing to 77% in 2004-2008. Virological and immunological responses at 12 months varied by initial ART type (P < 0.001 and P = 0.03, respectively), with four-drug nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens being superior [virological response <400 copies/ml adjusted odds ratio = 3.00, 95% confidence interval (CI) 1.24-7.23; mean increase in CD4 Z-score coefficient = 0.64, 95% CI 0.10-1.17] to both three-drug NNRTI-based (reference) and boosted protease inhibitor regimens which were similar. Rates of switching to second-line ART were lower among children starting four-drug NNRTI-based and boosted protease inhibitor-based regimens compared with three-drug NNRTI regimens (P = 0.03). Sixty five percent of infants remained on first-line ART without treatment interruption after 5 years.
Conclusion: Effective and prolonged responses to first-line ART can now be achieved in infants starting early ART outside trial settings. Superior responses to four-drug NNRTI compared with boosted protease inhibitor or three-drug NNRTI regimens need further evaluation, as does treatment interruption following early ART.
2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Figures
References
-
- World Health Organization . Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. World Health Organization; Geneva: 2010.
-
- Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23:597–604. - PubMed
-
- Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22(11):1333–43. - PubMed
-
- Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20(2):207–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

