Rhodium-catalyzed annulation of N-benzoylsulfonamide with isocyanide through C-H activation
- PMID: 21972033
- PMCID: PMC3426457
- DOI: 10.1002/chem.201102475
Rhodium-catalyzed annulation of N-benzoylsulfonamide with isocyanide through C-H activation
Abstract
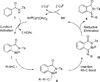
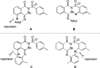
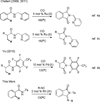
Isocyanide insertion: the first rhodium-catalyzed annulation of N-benzoylsulfonamide incorporating with isocyanide via C-H activation is described. The transformation is broadly compatible with N-benzoylsulfonamides bearing various electron-properties as well as isocyanides. From practical point of view, this methodology provides the most straightforward approach to a series of 3-(imino)isoindolinones.
Figures
References
-
-
For recent reviews: Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. Ackermann L. Chem. Commun. 2010;46:4866. Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. and references cited therein.
-
-
-
For examples using alkenes: Miura M, Tsuda T, Satoh T, Pivsa-Art S, Nomura M. J. Org. Chem. 1998;63:5211. Houlden CE, Bailey CD, Ford JG, Gagné MR, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2008;130:10066. Li J-J, Mei T-S, Yu J-Q. Angew. Chem., Int. Ed. 2008;47:6452. Wasa M, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2010;132:3680. Wang F, Song G, Li X. Org. Lett. 2010;12:5430. Rakshit S, Grohmann C, Besset T, Glorius F. J. Am. Chem. Soc. 2011;133:2350.
-
-
-
For examples using alkynes: Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J. Am. Chem. Soc. 2008;130:16474. Guimond N, Gouliaras C, Fagnou K. J. Am. Chem. Soc. 2010;132:6908. Rakshit S, Patureau FW, Glorius F. J. Am. Chem. Soc. 2010;132:9585. Hyster TK, Rovis T. J. Am. Chem. Soc. 2010;132:10565. Stuart DR, Alsabeh P, Kuhn M, Fagnou K. J. Am. Chem. Soc. 2010;132:18326. Mochida S, Umeda N, Hirano K, Satoh T, Miura M. Chem. Lett. 2010;39:744. Song G, Chen D, Pan C-L, Crabtree RH, Li X. J. Org. Chem. 2010;75:7487. Su Y, Zhao M, Han K, Song G, Li X. Org. Lett. 2010;12:5462. Ackermann L, Lygin AV, Hofmann N. Org. Lett. 2011;13:3278. Ackermann L, Lygin AV, Hofmann N. Angew. Chem., Int. Ed. 2011;50:6379. Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449.
-
-
-
For recent reviews: Dömling A. Chem. Rev. 2006;106:17. Kaim LE, Grimaud L. Tetrahedron. 2009;65:2153. Banfi L, Riva R, Basso A. Synlett. 2010:23. Lygin AV, de Meijere A. Angew. Chem., Int. Ed. 2010;49:9094. Tobisu M, Chatani N. Chem. Lett. 2011;40:330. and references cited therein.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources