Addition of iron to erythropoiesis-stimulating agents in cancer patients: a meta-analysis of randomized trials
- PMID: 21972052
- PMCID: PMC11824565
- DOI: 10.1007/s00432-011-1072-3
Addition of iron to erythropoiesis-stimulating agents in cancer patients: a meta-analysis of randomized trials
Abstract
Background: Iron supplementation could improve the hematopoietic response of erythropoiesis-stimulating agents (ESAs) used for chemotherapy-induced anemia.
Methods: We performed a meta-analysis of randomized, controlled trials by comparing parenteral or oral iron and no iron, when added to ESAs in anemic cancer patients, in order to calculate the relative risk (RR) of hematopoietic response and transfusions, the time required to reach this response, and toxicity.
Results: A total of 1,606 patients out of eight trials were available for meta-analysis. The RR of obtaining an hematopoietic response was 1.29 (P = 0.0001) with parenteral iron and 1.04 for oral iron (P = 0.59). The risk of transfusion was reduced with parenteral iron versus no iron (RR 0.77; P = 0.02) but not with oral iron (RR 0.68; P = 0.08). The time to reach hematopoietic response was 1 month shorter and no increased toxicity appeared with iron supplementation.
Conclusion: Overall parenteral iron reduces the risk of transfusions by 23% and increases the chance of hematopoietic response by 29% when compared with ESAs alone. On the contrary, oral iron does not increase hematopoietic response nor transfusion rate. The significance of these results is that the proportion of non-responders to ESAs will have strongly improved and quality of life and cost ameliorated.
Figures
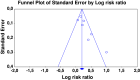
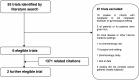



Comment in
-
Hematology: cast iron results for ESA use in cancer.Nat Rev Clin Oncol. 2011 Nov 1;8(12):691. doi: 10.1038/nrclinonc.2011.166. Nat Rev Clin Oncol. 2011. PMID: 22048627 No abstract available.
References
-
- Demetri GD, Kris M, Wade J et al (1998) Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol 16:3412–3425 - PubMed
-
- Gabrilove JL, Cleeland CS, Livingston RB et al (2001) Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol 19:2875–2882 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

